| Pharmaceutical Information |
| Drug Name |
Zinc oxide |
| Drug ID |
BADD_D02642 |
| Description |
Zinc oxide is an inorganic compound used in a number of manufacturing processes. It can be found in rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. It occurs naturally as the mineral zincite, but most zinc oxide is produced synthetically. It is also widely used to treat a variety of other skin conditions, in products such as baby powder and barrier creams to treat diaper rashes, calamine cream, anti-dandruff shampoos, and antiseptic ointments. |
| Indications and Usage |
For adjunctive treatment of diaper dermatitis. Also, it can be used to treat minor skin irritations (eg, cuts, burns, and scrapes, poison ivy).
Zinc oxide can be used in ointments, creams, and lotions to protect against sunburn and other damage to the skin caused by ultraviolet light. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09321
|
| KEGG ID |
D01170
|
| MeSH ID |
D015034
|
| PubChem ID |
14806
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
72805-671; 72812-147; 72812-148; 72841-101; 72962-101; 73022-005; 73054-108; 73054-126; 73088-301; 73122-071; 73369-1001; 73369-1206; 73381-506; 73572-003; 73618-001; 75071-415; 75936-149; 76144-776; 76150-331; 76348-676; 76354-473; 76354-474; 76354-492; 76354-493; 79753-050; 81110-055; 81110-057; 81110-760; 81310-006; 81540-060; 81929-002; 82114-001; 82495-101; 82635-403; 82763-1020; 83046-357; 83100-1720; 83108-001; 83108-002; 83108-040; 83241-1039; 83252-004; 90086-010; 10117-3006; 49354-106; 49354-107; 49354-118; 54088-203; 61503-203; 61726-101; 69253-001; 69912-012; 70979-502; 71028-1102; 71028-2207; 73139-105; 73174-050; 77793-017; 80466-121; 81407-001; 11673-226; 36800-251; 42508-476; 42508-479; 42851-091; 51326-130; 51785-900; 51861-080; 52358-0004; 52854-615; 53329-220; 54136-153; 55165-0201; 55165-1006; 55165-1007; 58232-9915; 58518-010; 0316-2083; 59958-240; 60781-2018; 60934-013; 61197-302; 61354-114; 61649-034; 61649-120; 62206-3031; 62206-4710; 62206-4714; 62206-4760; 62654-145; 0363-0994; 63354-820; 63931-1158; 63931-4302; 63931-4338; 63931-4372; 63931-4441; 63931-4477; 63931-4479; 63931-4506; 63931-4516; 63931-4543; 64768-2926; 68078-033; 69026-499; 69039-623; 69366-224; 69417-130; 69546-322; 69740-324; 69740-325; 69949-010; 69949-251; 69968-0395; 69968-0476; 69968-0547; 69968-0549; 69968-0602; 69968-0635; 69968-0677; 69968-0739; 69968-0775; 70631-137; 70668-827; 70680-301; 70680-303; 70680-306; 70793-026; 70793-033; 70793-035; 70983-021; 71047-364; 71182-0002; 71182-0005; 71182-0006; 71182-0009; 71499-045; 71644-014; 71780-520; 72098-004; 72203-016; 72209-001; 72667-049; 72667-050; 72667-052; 73122-061; 73122-063; 73369-0006; 73369-1003; 73418-020; 73517-427; 74154-318; 75786-224; 75936-158; 75936-223; 75936-618; 76209-001; 77394-202; 79265-8063; 79265-9606; 79753-009; 79753-057; 80325-000; 80651-093; 81110-757; 81899-005; 81899-040; 81899-050; 81899-065; 82086-002; 82088-131; 82184-1173; 82184-4480; 82350-352; 82520-263; 82520-264; 82526-269; 82548-4530; 82575-034; 82657-004; 82751-101; 82769-100; 82870-005; 83046-371; 83252-006; 83252-007; 83578-205; 90086-005; 10117-3008; 42944-101; 49354-122; 54088-207; 60396-1001; 60396-1006; 61503-319; 10356-115; 63931-4489; 66240-306; 67278-2100; 69912-007; 71944-0003; 72955-302; 76002-0140; 77793-014; 80466-115; 80466-119; 16864-021; 26052-493; 35192-050; 0132-0194; 37808-020; 37808-039; 42508-827; 45334-332; 45634-469; 45634-720; 46122-676; 52410-3040; 52412-400; 52915-140; 54108-0420; 54108-0423; 54111-139; 55165-0111; 55165-1001; 55165-1005; 55301-220; 55681-304; 58133-652; 59088-563; 0316-2011; 0316-2081; 60000-020; 61296-015; 61354-048; 61354-113; 61481-0036; 61649-043; 61649-068; 62206-2850; 62206-4023; 62206-4713; 62742-4114; 62742-4209; 63931-1657; 63931-1803; 63931-4348; 63931-4363; 63931-4377; 63931-4413; 63931-4416; 63931-4438; 63931-4442; 63931-4443; 63931-4454; 63931-4463; 63931-4464; 63931-4473; 63931-4486; 63931-4505; 63931-4572; 63941-318; 64024-020; 66163-1001; 67234-001; 68196-002; 68599-0205; 69026-483; 69026-488; 69039-255; 69039-275; 69039-622; 69204-028; 69423-621; 69423-622; 69423-692; 69435-1501; 69455-964; 69555-045; 69842-058; 69842-468; 69842-481; 69968-0275; 69968-0551; 69968-0583; 69968-0613; 69968-0623; 70170-0016; 70477-030; 70604-030; 70680-302; 70793-010; 70793-032; 72098-006; 72381-101; 72667-051; 72805-669; 72849-101; 73054-107; 73122-069 |
| UNII |
SOI2LOH54Z
|
| Synonyms |
Zinc Oxide | Oxide, Zinc | Lassar's Paste | Lassar Paste | Lassars Paste | Paste, Lassar's |
|
| Chemical Information |
| Molecular Formula |
OZn |
| CAS Registry Number |
1314-13-2 |
| SMILES |
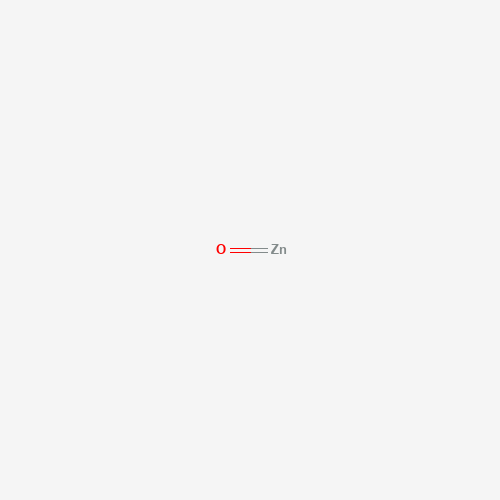
O=[Zn] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

