| Pharmaceutical Information |
| Drug Name |
Benzethonium |
| Drug ID |
BADD_D02614 |
| Description |
Benzethonium is a synthetic quaternary ammonium salt with surfactant, antiseptic, and broad spectrum antimicrobial properties. Its salt form, benzethonium chloride, is primarily used as a skin disinfectant at concentrations of 0.1-0.2 %, which are safe and effective concentrations for the compound specified by the U.S. Food and Drug Administration (FDA). It is additionally found in cosmetics and toiletries such as mouthwashes and anti-itch ointments. It is shown to be effective in mediating its antimicrobial action against bacteria, fungi, mold and viruses. There is evidence that benzethonium acts as a spermatocide but may cause vaginal irritation [A32304]. Benzethonium was identified as a novel cancer-specific compound by cell-based small-molecule screen [A32298]. |
| Indications and Usage |
Indicated as an antiseptic agent. No therapeutic indications for clinical use. |
| Marketing Status |
approved |
| ATC Code |
R02AA09 |
| DrugBank ID |
DB11125
|
| KEGG ID |
D01140
|
| MeSH ID |
D001558
|
| PubChem ID |
2335
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
1VU15B70BP
|
| Synonyms |
Benzethonium | Orchid Fresh II | Formula Magic | Magic, Formula | Hyamine 1622 | Solamin | Phemerol | Phemethryn | Puri-Clens | Puri Clens | PuriClens | Quatrachlor | Benzethonium Chloride | Chloride, Benzethonium | Bencetonium Chloride | Chloride, Bencetonium | Phemeride |
|
| Chemical Information |
| Molecular Formula |
C27H42NO2+ |
| CAS Registry Number |
10172-60-8 |
| SMILES |
CC(C)(C)CC(C)(C)C1=CC=C(C=C1)OCCOCC[N+](C)(C)CC2=CC=CC=C2 |
| Chemical Structure |
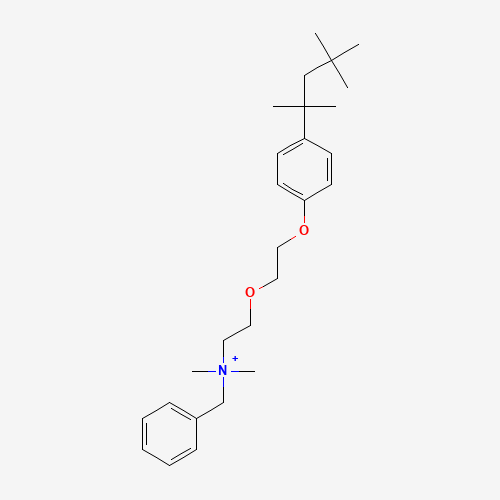
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

