| Pharmaceutical Information |
| Drug Name |
Tepotinib |
| Drug ID |
BADD_D02599 |
| Description |
Tepotinib is a MET tyrosine kinase inhibitor intended to treat a variety of MET-overexpressing solid tumors.[A228058] It was originally developed in partnership between EMD Serono and the University of Texas M.D. Anderson Cancer Center in 2009 and has since been investigated in the treatment of neuroblastoma,[A228053] gastric cancers,[A228033] non-small cell lung cancer, and hepatocellular carcinoma.[A228058] MET is a desirable target in the treatment of certain solid tumors as it appears to play a critical role, both directly and indirectly, in the growth and proliferation of tumors in which it is overexpressed and/or mutated.
Tepotinib was first approved in Japan in March 2020 for the treatment of non-small cell lung cancers (NSCLC) with _MET_ alterations, and was subsequently granted accelerated approval by the US FDA in February 2021, under the brand name Tepmetko, for the treatment of adult patients with metastatic NSCLC and _MET_ exon 14 skipping alterations.[L31443,L31473] It is the first oral MET-targeted tyrosine kinase inhibitor to allow for once-daily dosing,[L31473] an advantage that may aid in easing the pill burden often associated with chemotherapeutic regimens. |
| Indications and Usage |
Tepotinib is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) who have mesenchymal-epithelial transition (_MET_) exon 14 skipping alterations.[L31443] |
| Marketing Status |
approved; investigational |
| ATC Code |
L01EX21 |
| DrugBank ID |
DB15133
|
| KEGG ID |
D11717
|
| MeSH ID |
C000707607
|
| PubChem ID |
25171648
|
| TTD Drug ID |
D0R8QG
|
| NDC Product Code |
Not Available |
| UNII |
1IJV77EI07
|
| Synonyms |
tepotinib | 3-(1-(3-(5-((1-Methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)benzyl)-6-oxo-1,6-dihydro-3-pyridazinyl)benzonitrile | benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo-3-pyridazinyl)- | tepmetko |
|
| Chemical Information |
| Molecular Formula |
C29H28N6O2 |
| CAS Registry Number |
1100598-32-0 |
| SMILES |
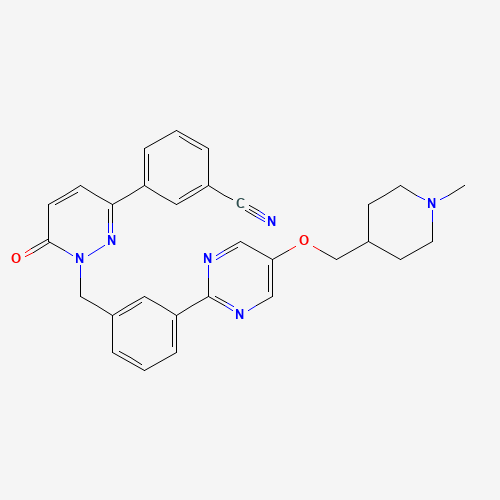
CN1CCC(CC1)COC2=CN=C(N=C2)C3=CC=CC(=C3)CN4C(=O)C=CC(=N4)C5=CC=CC(=C5)C#N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

