| Pharmaceutical Information |
| Drug Name |
Glasdegib |
| Drug ID |
BADD_D02559 |
| Description |
Glasdegib, also known as PF-04449913, is a small-molecule hedgehog signaling inhibitor selected under the group of the benzimidazoles. In early research, benzimidazoles attracted large interest as they represented a class of inhibitors with a low molecular weight, potent inhibitory activity and lacking unstable functionality. The great lipophilicity of this group of compounds brought interest to further modification. This analysis concluded that the presence of p-cyano ureas presented good physicochemical and pharmacokinetic properties from which glasdegib was developed.[A40310]
Glasdegib was developed by Pfizer Inc and approved on November 21, 2018, by the FDA for the treatment of Acute Myeloid Leukemia.[L11935] |
| Indications and Usage |
Glasdegib, in combination with cytarabine, is indicated for the treatment of newly diagnosed acute myeloid leukemia in adult patients who are over 75 years old or that have co-morbidities that preclude intensive induction chemotherapy.[L5080]
Acute myeloid leukemia is characterized by abnormal production of myeloblasts, red cells, or platelets. It is considered a cancer of blood and bone marrow and it is the most common type of acute leukemia in adults.[L4832] |
| Marketing Status |
approved; investigational |
| ATC Code |
L01XJ03 |
| DrugBank ID |
DB11978
|
| KEGG ID |
D10636
|
| MeSH ID |
C000592580
|
| PubChem ID |
25166913
|
| TTD Drug ID |
D0S5LO
|
| NDC Product Code |
0069-0298; 0069-1531 |
| UNII |
K673DMO5H9
|
| Synonyms |
glasdegib | Daurismo | 1-(2-(1H-benzo(d)imidazol-2-yl)-1-methylpiperidin-4-yl)-3-(4-cyanophenyl)urea | PF-04449913 |
|
| Chemical Information |
| Molecular Formula |
C21H22N6O |
| CAS Registry Number |
1095173-27-5 |
| SMILES |
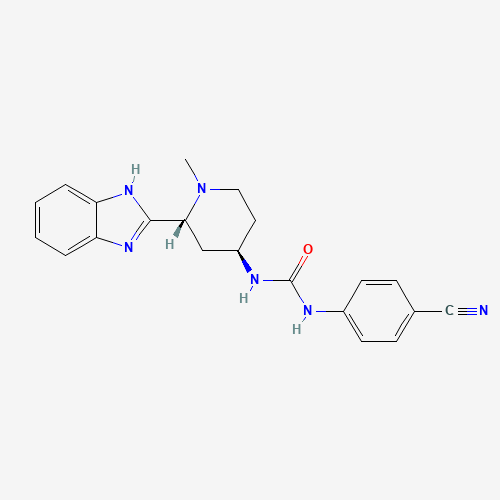
CN1CCC(CC1C2=NC3=CC=CC=C3N2)NC(=O)NC4=CC=C(C=C4)C#N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

