| Pharmaceutical Information |
| Drug Name |
Tinzaparin sodium |
| Drug ID |
BADD_D02222 |
| Description |
Tinzaparin is a low molecular weight heparin (LMWH), produced by enzymatic depolymerization of unfractionated heparin from porcine intestinal mucosa. It is a heterogeneous mixture of with an average molecular weight between 5500 and 7500 daltons. Tinzaparin is composed of molecules with and without a special site for high affinity binding to antithrombin III (ATIII). This complex greatly accelerates the inhibition of factor Xa. It is an anticoagulant and considered an antithrombotic. Tinzaparin must be given either subcutaneously or parenterally. LMWHs are less effective at inactivating factor IIa due to their shorter length compared to unfractionated heparin. |
| Indications and Usage |
Tinzaparin is used for the prevention of postoperative venous thromboembolism in patients undergoing orthopedic surgery and in patients undergoing general surgery who are at high risk of developing postoperative venous thromboembolism. It is also used for the treatment of deep vein thrombosis and/or pulmonary embolism. It is indicated for use in preventing clot formation in indwelling intravenous lines for hemodialysis. |
| Marketing Status |
approved |
| ATC Code |
B01AB10 |
| DrugBank ID |
DB06822
|
| KEGG ID |
D06398
|
| MeSH ID |
D000078222
|
| PubChem ID |
8784
|
| TTD Drug ID |
D01ZJK
|
| NDC Product Code |
Not Available |
| UNII |
3S182ET3UA
|
| Synonyms |
Tinzaparin | 2-Propenoic acid, 3-phenyl- | 2 Propenoic acid, 3 phenyl | 3-phenyl- 2-Propenoic acid | Tinzaparin Sodium | Innohep |
|
| Chemical Information |
| Molecular Formula |
C9H8O2 |
| CAS Registry Number |
621-82-9 |
| SMILES |
C1=CC=C(C=C1)C=CC(=O)O |
| Chemical Structure |
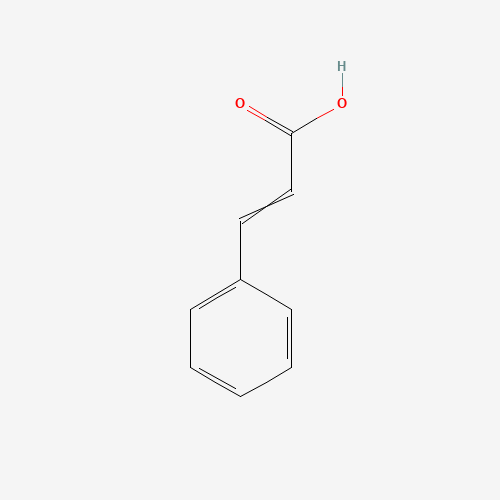
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

