| Pharmaceutical Information |
| Drug Name |
Rotigotine hydrochloride |
| Drug ID |
BADD_D01974 |
| Description |
Rotigotine (Neupro) is a non-ergoline dopamine agonist indicated for the treatment of Parkinson's disease (PD) and restless legs syndrome (RLS) in Europe and the United States. It is formulated as a once-daily transdermal patch which provides a slow and constant supply of the drug over the course of 24 hours.
Like other dopamine agonists, rotigotine has been shown to possess antidepressant effects and may be useful in the treatment of depression as well.
Rotigotine was developed by Aderis Pharmaceuticals. In 1998 Aderis licensed worldwide development and commercialization rights to Schwarz Pharma of Germany. It was approved by the European Medicines Agency in 2006 and by the FDA in 2007. However, all Neupro patches in the United States and some of Europe were recalled in 2008 due to delivery mechanism issues. Rotigotine has been authorized as a treatment for RLS since August 2008. |
| Indications and Usage |
For use/treatment in neurologic disorders and parkinson's disease as well as moderate-to-severe primary Restless Legs Syndrome. |
| Marketing Status |
approved |
| ATC Code |
N04BC09 |
| DrugBank ID |
DB05271
|
| KEGG ID |
D05768
|
| MeSH ID |
C047508
|
| PubChem ID |
180335
|
| TTD Drug ID |
D0E4JL
|
| NDC Product Code |
17337-0078 |
| UNII |
6Q1W9573L2
|
| Synonyms |
rotigotine | 2-(N-n-propyl-N-2-thienylethylamino)-5-hydroxytetralin | N 0437, hydrochloride, (S)-isomer | N 0923 | N-0923 | N 0924 | N-0924 | rotigotine, (+)- | (+)-5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol | Neupro | N 0437 | N-0437 | N 0437, (+-)-isomer | N 0437, (-)-isomer | N 0437, hydrochloride, (R)-isomer | rotigotine, (+--)- | racemic N-0437 | rotigotine (+-)-form | 1-naphthalenol, 5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)- | (+--)-5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol | N 0437, (R)-isomer | Rotigotine CDS |
|
| Chemical Information |
| Molecular Formula |
C19H26ClNOS |
| CAS Registry Number |
125572-93-2 |
| SMILES |
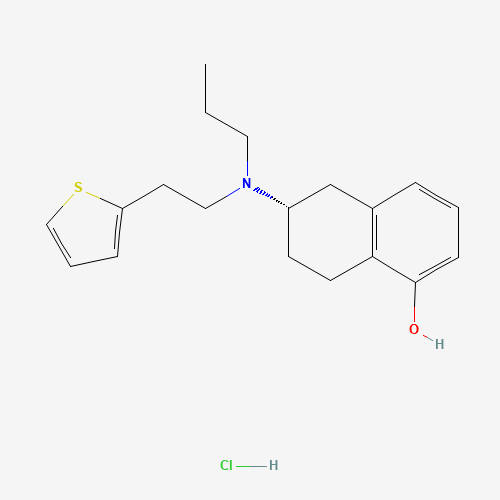
CCCN(CCC1=CC=CS1)C2CCC3=C(C2)C=CC=C3O.Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

