| Pharmaceutical Information |
| Drug Name |
Ketorolac |
| Drug ID |
BADD_D01232 |
| Description |
Ketorolac is a Non-steroidal anti-inflammatory drug (NSAID) and is commercially available as an oral tablet, injectable, nasal spray and as an ophthalmic solution.[L6358][L6508][L11055][L3674][L11070]
It's analgesic properties make it a useful pain management tool across many settings including postoperative pain, rheumatoid arthritis, osteoarthritis, menstrual disorders, headaches, spinal and soft tissue pain, and ankylosing spondylitis.[L6520] Impressively, ketorolac has a similar efficacy to standard doses of morphine and meperidine making it a useful opioid sparing agent.[L6511] |
| Indications and Usage |
Ketorolac is a Non-steroidal anti-inflammatory drug (NSAID) and has antipyretic, analgesic and anti-inflammatory properties.[A176131] It is indicated for short term management of acute pain that requires the calibre of pain management offered by opioids.[L3674] Clinicians may choose to initiate ketorolac to manage post-operative pain, spinal and soft tissue pain, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, menstrual disorders and headaches among other ailments.[L6520] Regardless of the etiology of pain, patients should use the lowest possible dose, and avoid using ketorolac for an extended period of time (ideally ≤ 5 days).[L3674] A benefit of choosing ketorolac over other analgesics with similar potency is that that there does not appear to be a risk of dependence or tolerance with ketorolac use.[A176131] |
| Marketing Status |
approved |
| ATC Code |
M01AB15; S01BC05 |
| DrugBank ID |
DB00465
|
| KEGG ID |
D08104
|
| MeSH ID |
D020910
|
| PubChem ID |
3826
|
| TTD Drug ID |
D0D9JW
|
| NDC Product Code |
Not Available |
| UNII |
YZI5105V0L
|
| Synonyms |
Ketorolac |
|
| Chemical Information |
| Molecular Formula |
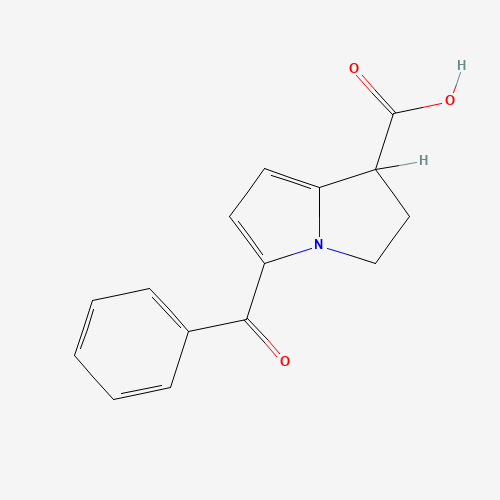
C15H13NO3 |
| CAS Registry Number |
74103-06-3 |
| SMILES |
C1CN2C(=CC=C2C(=O)C3=CC=CC=C3)C1C(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

