| Pharmaceutical Information |
| Drug Name |
Hyaluronidase |
| Drug ID |
BADD_D01078 |
| Description |
Hyaluronidase is an enzyme used to improve the absorption and dispersion of parenterally administered fluids, drugs, and contrast agents.[L13338] The action of hyaluronidase was first described in 1936, and named in 1939.[A199026] Early research into hyaluronidase identified it as a "spreading factor" which allowed for increased permeability of the connective tissue.[A199026] Hyaluronidase has been used in surgical settings for at least the past 60 years to improve the diffusion of local anesthetics.[A199047]
Hyaluronidase was first used in prescription products in the United States on 5 May 2004.[L13338] |
| Indications and Usage |
Hyaluronidase is indicated for subcutaneous fluid administration for hydration, and increasing resorption of radiopaque agents in subcutaneous urography.[L13338] Hyaluronidase is also indicated by multiple routes to increase the dispersion of other injectable drugs.[L13338] |
| Marketing Status |
approved |
| ATC Code |
B06AA03 |
| DrugBank ID |
DB14740
|
| KEGG ID |
D04455; D04456; D06604
|
| MeSH ID |
D006821
|
| PubChem ID |
91820602
|
| TTD Drug ID |
D04GPZ
|
| NDC Product Code |
0548-9090; 51662-1555; 52221-201 |
| UNII |
Not Available
|
| Synonyms |
Hyaluronoglucosaminidase | Hyaluronidase | Hyaglosidase | Hyaluronate Hydrolase | Hydrolase, Hyaluronate | Duran-Reynals Permeability Factor | Duran Reynals Permeability Factor | Factor, Duran-Reynals Permeability | Permeability Factor, Duran-Reynals | GL Enzyme | Wydase |
|
| Chemical Information |
| Molecular Formula |
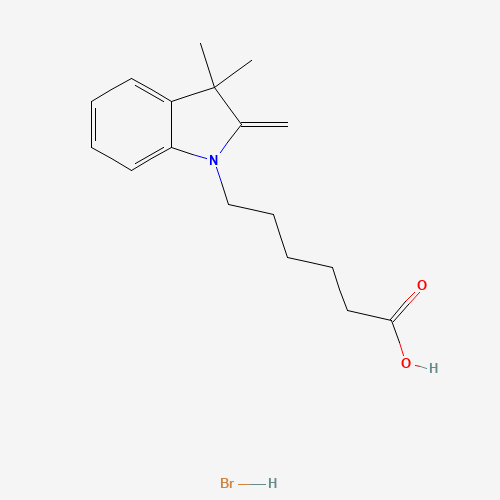
C17H24BrNO2 |
| CAS Registry Number |
9001-54-1 |
| SMILES |
CC1(C(=C)N(C2=CC=CC=C21)CCCCCC(=O)O)C.Br |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Anaphylactic reaction | 10.01.07.001; 24.06.03.006 | - | - | | | Angioedema | 10.01.05.009; 22.04.02.008; 23.04.01.001 | - | - | - | | Oedema | 08.01.07.006; 14.05.06.010 | - | - | - | | Urticaria | 10.01.06.001; 23.04.02.001 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

