| Pharmaceutical Information |
| Drug Name |
Celiprolol |
| Drug ID |
BADD_D00411 |
| Description |
Celiprolol is indicated for the management of mild to moderate hypertension and effort-induced angina pectoris. It is simultaneously a selective β1 receptor antagonist, a β2 receptor partial agonist and a weak α2 receptor antagonist. In 2010 a clinical trial has suggested a use for this medication in the prevention of vascular complications of a rare inherited disease called vascular Ehlers–Danlos syndrome. This study demonstrated decreased incidence of arterial rupture or dissection (a specific type of arterial rupture in which the layers of the vessel separate prior to complete failure of the artery wall). Celiprolol is not approved for use by the FDA in the treatment of vascular Ehlers–Danlos syndrome. |
| Indications and Usage |
Celiprolol is indicated for the management of mild to moderate hypertension and effort-induced angina pectoris. |
| Marketing Status |
approved; investigational |
| ATC Code |
C07AB08 |
| DrugBank ID |
DB04846
|
| KEGG ID |
D07660
|
| MeSH ID |
D017272
|
| PubChem ID |
2663
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
DRB57K47QC
|
| Synonyms |
Celiprolol | N'-(3-Acetyl-4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)phenyl)-N,N-diethylurea | Celiprolol, Monohydrochloride, (R)-Isomer | Celiprolol, Monohydrochloride, (S)-Isomer | REV-5320A | REV 5320A | REV5320A | Celiprolol, (R)-Isomer | ST-1396 | ST 1396 | ST1396 | Celiprolol Hydrochloride | Hydrochloride, Celiprolol | Celiprolol Monohydrochloride | Monohydrochloride, Celiprolol | Celiprolol, (+,-)-Isomer | Celiprolol, (S)-Isomer | Selectol |
|
| Chemical Information |
| Molecular Formula |
C20H33N3O4 |
| CAS Registry Number |
56980-93-9 |
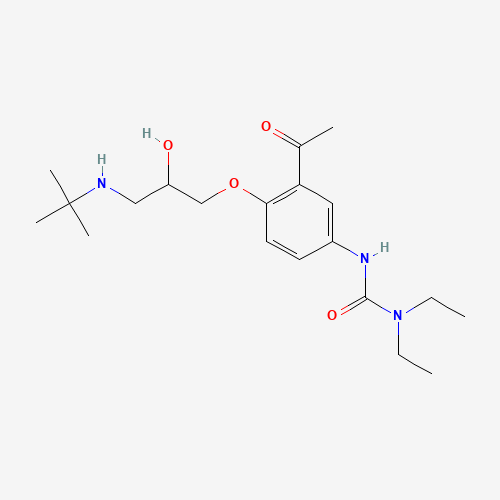
| SMILES |
CCN(CC)C(=O)NC1=CC(=C(C=C1)OCC(CNC(C)(C)C)O)C(=O)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

