| Pharmaceutical Information |
| Drug Name |
Bremelanotide |
| Drug ID |
BADD_D02536 |
| Description |
Bremelanotide is a 7 amino acid peptide used to treat hypoactive sexual desire disorder in premenopausal women.[L9635] Bremelanotide does not interact with alcohol.[A179686] The mechanism by which bremelanotide's action on receptors translates to a clinical effect is still unknown.[L9635]
Bremelanotide was first described in the literature in 2003 when it was known by the investigational code PT-141.[A179683] Since then it was investigated for its place in treating sexual dysfunction in men and women but is now only indicated for women.[A179683,A179686,L9635] Other drugs used to treat female sexual dysfunction include [flibanserin], [estrogen], [ospemifene], and [prasterone].[A179689]
Bremelanotide was granted FDA approval on 21 June 2019.[L9635] |
| Indications and Usage |
Bremelanotide is indicated to treat premenopausal women with hypoactive sexual desire disorder that is not due to a medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug.[L9635] |
| Marketing Status |
approved; investigational |
| ATC Code |
G02CX05 |
| DrugBank ID |
DB11653
|
| KEGG ID |
D06569
|
| MeSH ID |
C476721
|
| PubChem ID |
9941379
|
| TTD Drug ID |
D0X9PF
|
| NDC Product Code |
80064-141; 43835-0025 |
| UNII |
6Y24O4F92S
|
| Synonyms |
bremelanotide | Vyleesi | PT-141 |
|
| Chemical Information |
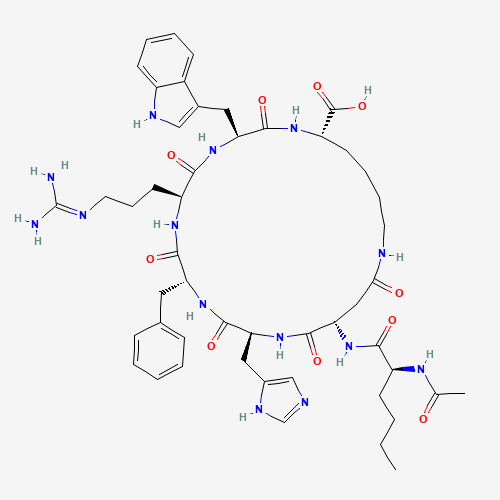
| Molecular Formula |
C50H68N14O10 |
| CAS Registry Number |
189691-06-3 |
| SMILES |
CCCCC(C(=O)NC1CC(=O)NCCCCC(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC1=O)CC2=CN=CN2)CC3=
CC=CC=C3)CCCN=C(N)N)CC4=CNC5=CC=CC=C54)C(=O)O)NC(=O)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Drug ineffective | 08.06.01.006 | 0.000276% | | - | | Flushing | 08.01.03.025; 23.06.05.003; 24.03.01.002 | 0.000484% | | | | Headache | 17.14.01.001 | 0.000435% | | | | Injection site erythema | 08.02.03.001; 12.07.03.001; 23.03.06.015 | 0.000138% | | - | | Insomnia | 17.15.03.002; 19.02.01.002 | 0.000138% | | | | Nausea | 07.01.07.001 | 0.000919% | | | | Vomiting | 07.01.07.003 | 0.000228% | | | | Illness | 08.01.03.091 | 0.000138% | | - |
|
The 1th Page
1
Total 1 Pages
|
|

