| Pharmaceutical Information |
| Drug Name |
Gilteritinib |
| Drug ID |
BADD_D02505 |
| Description |
Gilteritinib, also known as ASP2215, is a small molecule part of the FLT3 tyrosine kinase inhibitors that presented a greater selectivity and potency when compared with other agents from this group.[A40036] It is a pyrazinecarboxamide derivative that showed high selectivity to FLT3 preventing the c-Kit -driven myelosuppression observed in other therapies.[A40044] Gilteritinib was developed by Astellas Pharma and FDA approved on November 28, 2018. This drug was approved after being designed as an orphan drug with a fast track and priority review status.[L4830] |
| Indications and Usage |
Gilteritinib is indicated for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia with an FLT3 mutation detected by an FDA-approved test. This indication was expanded for a companion diagnostic to include use with gilteritinib such as the LeukoStrat CDx FLT3 Mutation Assay.[L4830]
Acute myeloid leukemia is cancer that impacts the blood and bone marrow with a rapid progression. This condition produces low numbers of normal blood cells and the requirement of continuous need for transfusions.[L4832] |
| Marketing Status |
approved; investigational |
| ATC Code |
L01EX13 |
| DrugBank ID |
DB12141
|
| KEGG ID |
D10709
|
| MeSH ID |
C000609080
|
| PubChem ID |
49803313
|
| TTD Drug ID |
D04KZY
|
| NDC Product Code |
0469-1425 |
| UNII |
66D92MGC8M
|
| Synonyms |
gilteritinib | ASP-2215 | ASP2215 | Xospata |
|
| Chemical Information |
| Molecular Formula |
C29H44N8O3 |
| CAS Registry Number |
1254053-43-4 |
| SMILES |
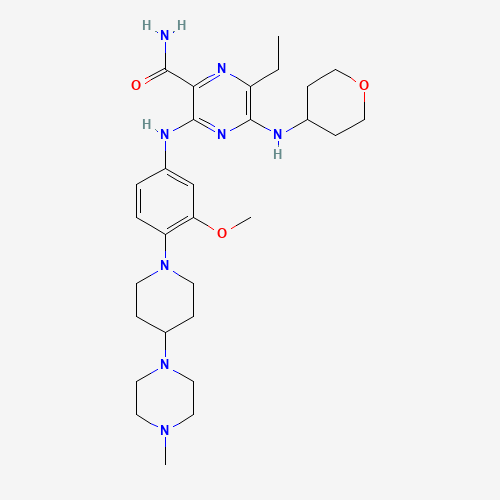
CCC1=C(N=C(C(=N1)C(=O)N)NC2=CC(=C(C=C2)N3CCC(CC3)N4CCN(CC4)C)OC)NC5CCOCC5 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

