| Pharmaceutical Information |
| Drug Name |
Zutracin |
| Drug ID |
BADD_D02399 |
| Description |
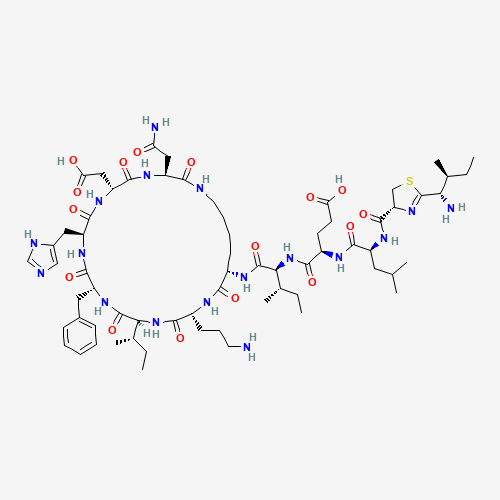
Bacitracin is a combination of at least 9 bacitracins.[A955,A181952] 60-80% of commercially prepared bacitracin is bacitracin A.[A181952] The bacillus that produces bacitracin was first isolated from a knee scrape in 1945 from the knee wound of a child named Margaret Tracy.[A181952]
Bacitracin was granted FDA approval on 29 July 1948.[A181997,L7748] |
| Indications and Usage |
Bacitracin is indicated in topical formulations for acute and chronic localized skin infections.[A181997] Occasionally, it is also used intramuscularly for infantile streptococcal pneumonia and empyema.[A181997] Bacitracin is also formulated as an ointment with neomycin and polymyxin B for over the counter use.[A181997,L7769] A bacitracin ointment formulated with neomycin and polymyxin B along with hydrocortisone is indicated for the treatment of corticosteroid responsive dermatoses with secondary infection.[L7772] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
R02AB04; D06AX05; J01XX10 |
| DrugBank ID |
DB00626
|
| KEGG ID |
D00128; D03048
|
| MeSH ID |
D001414
|
| PubChem ID |
10909430
|
| TTD Drug ID |
D0M1IO
|
| NDC Product Code |
38779-0015; 55500-0002; 63187-299; 21130-757; 39892-0810; 59428-135; 0363-0075; 51551-0301; 68788-9794; 55910-536; 61919-116; 68001-477; 68071-4285; 71205-057; 0713-0280; 52928-001; 50090-0845; 55681-218; 67777-116; 68071-5253; 75983-005; 51552-1315; 76003-0162; 50090-0815; 67234-059; 30142-536; 36800-536; 0536-1256; 39892-0830; 45802-060; 59779-536; 59898-720; 80489-006; 11673-073; 41250-844; 0574-4022; 37808-536 |
| UNII |
DDA3RRX0P7
|
| Synonyms |
Bacitracin | Altracin | Baci-IM | Baci IM | Zeba-Rx | Zeba Rx | Baci-Rx | Baci Rx | Topitracin | Bacitin | Bacitracin Zinc Complex | Zinc Bacitracin | Bacitracin, Zinc | Bacitracin Zinc | Zinc, Bacitracin | Bacitracine Martinet | Martinet, Bacitracine | Ocu-Tracin | Ocu Tracin | OcuTracin | Ak-Tracin | Ak Tracin | Baciguent |
|
| Chemical Information |
| Molecular Formula |
C66H103N17O16S |
| CAS Registry Number |
22601-59-8 |
| SMILES |
CCC(C)C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCCCCC(C(=O)NC(C(=O)N1)CCCN)NC(=O)C
(C(C)CC)NC(=O)C(CCC(=O)O)NC(=O)C(CC(C)C)NC(=O)C2CSC(=N2)C(C(C)CC)N)CC(=O)N)CC(=O
)O)CC3=CN=CN3)CC4=CC=CC=C4 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Albuminuria | 20.02.01.001 | - | - | - | | Azotaemia | 20.01.01.001 | - | - | - | | Dermatitis | 23.03.04.002 | - | - | - | | Nausea | 07.01.07.001 | - | - | | | Pain | 08.01.08.004 | - | - | | | Rash | 23.03.13.001 | - | - | - | | Vomiting | 07.01.07.003 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

