| Pharmaceutical Information |
| Drug Name |
Ulipristal |
| Drug ID |
BADD_D02312 |
| Description |
Ulipristal is a selective progesterone receptor modulator used for the purposes of emergency contraception (Ella) and for the treatment of uterine fibroids (Fibristal). It is a derivative of 19-norprogesterone and has both antagonistic and partial agonist activity at the progesterone receptor. It also binds to glucocorticoid receptor, however compared to mifepristone (a progesterone receptor antagonist), ulipristal is more tolerable and has lower glucocorticoid activity and better binding affinity.
Ulipristal is currently recommended as first line therapy for emergency contraception, due to improved efficacy and similar side effect profile as compared to the traditional use of levonorgestrel or the Yuzpe regimen. The exact mechanism of action for ulipristal is still currently debated, though there is evidence that it functions by inhibiting ovulation. A recent systematic review proclaimed that the majority of available evidence demonstrates an inhibitory effect on ovulation rather than a post-fertilization effect on the endometrium, which has been heavily debated due to ethical concerns related to abortion (Rosato et al, 2016). Nevertheless, current and ongoing research into the agent's mechanism of action as an emergency contraceptive continue to provide potentially plausible evidence that ulipristal may, in fact, elicit activity on the endometrium that prevents embryo implantation [A175372, A175375, A175378]. |
| Indications and Usage |
As the product Ella (available in Canada and the US), ulipristal is indicated for use as emergency contraception after unprotected intercourse or possible contraceptive failure when administered within 120 hours (5 days) after unprotected intercourse or a known or suspected contraceptive failure. As the product Fibristal (available in Canada), ulipristal is indicated for treatment of the signs and symptoms of uterine fibroids in adult women. |
| Marketing Status |
approved |
| ATC Code |
G03AD02; G03XB02 |
| DrugBank ID |
DB08867
|
| KEGG ID |
D09567
|
| MeSH ID |
C094854
|
| PubChem ID |
13559279
|
| TTD Drug ID |
D0V4WD
|
| NDC Product Code |
Not Available |
| UNII |
6J5J15Q2X8
|
| Synonyms |
ulipristal | 17alpha-acetoxy-11beta-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione | HRP 2000 | Hrp-2000 | RTI-3021-022 | VA-2914 | VA2914 | CDB 2914 | CDB-2914 | RTI-3021-012 |
|
| Chemical Information |
| Molecular Formula |
C28H35NO3 |
| CAS Registry Number |
159811-51-5 |
| SMILES |
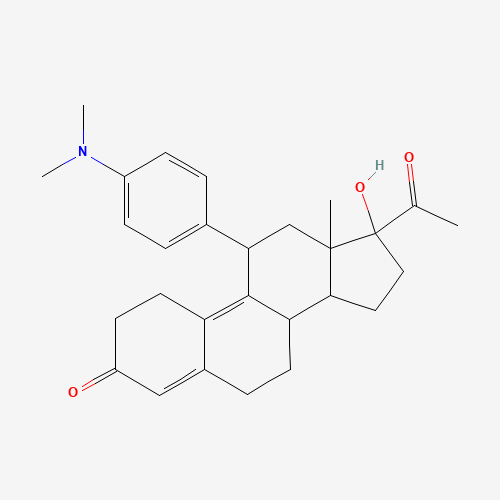
CC(=O)C1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

