| Pharmaceutical Information |
| Drug Name |
Tranexamic acid |
| Drug ID |
BADD_D02261 |
| Description |
Tranexamic acid is a synthetic derivative of [lysine] used as an antifibrinolytic in the treatment and prevention of major bleeding. It possesses a similar mechanism of action to [aminocaproic acid] but is approximately 10-fold more potent.[L31883]
It was first patented in 1957[A230108] and received its initial US approval in 1986.[L31858] |
| Indications and Usage |
Taken orally, tranexamic acid is indicated for the treatment of hereditary angioedema,[L31883] cyclic heavy menstrual bleeding in premenopausal females,[L31858] and other instances of significant bleeding in the context of hyperfibrinolysis.[L31883] Given intravenously, tranexamic acid is indicated for short-term use (2-8 days) in patients with hemophilia to prevent or reduce bleeding following tooth extraction.[L31853] |
| Marketing Status |
approved |
| ATC Code |
B02AA02 |
| DrugBank ID |
DB00302
|
| KEGG ID |
D01136
|
| MeSH ID |
D014148
|
| PubChem ID |
5526
|
| TTD Drug ID |
D05HXX
|
| NDC Product Code |
51927-0022; 73309-178; 42571-189; 50268-772; 69918-301; 70771-1085; 0591-3720; 49452-7876; 51552-0513; 81999-0001; 82920-038; 70121-1398; 23155-524; 43066-008; 55150-188; 63629-8838; 67850-041; 68083-160; 71335-1981; 60505-6169; 65145-106; 0517-0960; 81284-611; 12079-2001; 62991-3131; 82920-045; 51662-1532; 63629-8599; 67457-197; 70860-400; 70860-407; 71335-1957; 81284-612; 14537-115; 65388-0156; 61990-0611; 67850-042; 38779-3211; 57218-951; 25021-415; 42571-314; 50090-5072; 63323-563; 68382-891; 72611-760; 12848-1004; 0013-1114; 69918-300; 49452-7877; 71052-166; 23155-166; 51754-0108; 62559-265; 72485-107; 38779-2794; 39822-1000 |
| UNII |
6T84R30KC1
|
| Synonyms |
Tranexamic Acid | AMCHA | trans-4-(Aminomethyl)cyclohexanecarboxylic Acid | t-AMCHA | AMCA | Anvitoff | Cyklokapron | Ugurol | KABI 2161 | Spotof | Transamin | Amchafibrin | Exacyl |
|
| Chemical Information |
| Molecular Formula |
C8H15NO2 |
| CAS Registry Number |
701-54-2 |
| SMILES |
C1CC(CCC1CN)C(=O)O |
| Chemical Structure |
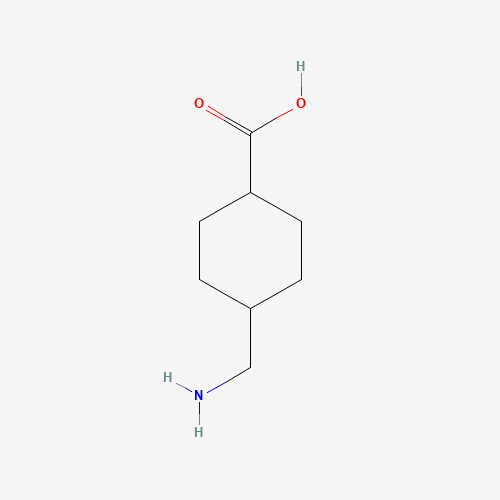
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

