| Pharmaceutical Information |
| Drug Name |
Thyrotropin alfa |
| Drug ID |
BADD_D02208 |
| Description |
Thyrotropin alfa is a recombinant form of thyroid stimulating hormone used in performing certain tests in patients who have or have had thyroid cancer. It is also used along with a radioactive agent to destroy remaining thyroid tissue in certain patients who have had their thyroid gland removed because of thyroid cancer. It is a heterodimeric glycoprotein comprised of two non-covalently linked subunits, an alpha subunit of 92 amino acid residues containing two N-linked glycosylation sites and a beta subunit of 112 residues containing one N-linked glycosylation site. The alpha subunit of thyrotropin alfa, which is the effector region responsible for the stimulation of adenylate cyclase, displays close structural similarity with the alpha subunit of human chorionic gonadotropin (hCG), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). The beta subunit (TSHB) bestows its receptor specificity due to the uniqueness to TSH. The amino acid sequence of thyrotropin alfa is identical to that of human pituitary thyroid stimulating hormone. |
| Indications and Usage |
For detection of residueal or recurrent thyroid cancer |
| Marketing Status |
approved; vet_approved |
| ATC Code |
H01AB01 |
| DrugBank ID |
DB00024
|
| KEGG ID |
D06120
|
| MeSH ID |
D057073
|
| PubChem ID |
32281
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
58468-0030 |
| UNII |
AVX3D5A4LM
|
| Synonyms |
Thyrotropin Alfa | Alfa, Thyrotropin | Recombinant Human TSH | Human TSH, Recombinant | TSH, Recombinant Human | rhTSH | Thyrogen |
|
| Chemical Information |
| Molecular Formula |
C16H22N6O4 |
| CAS Registry Number |
194100-83-9 |
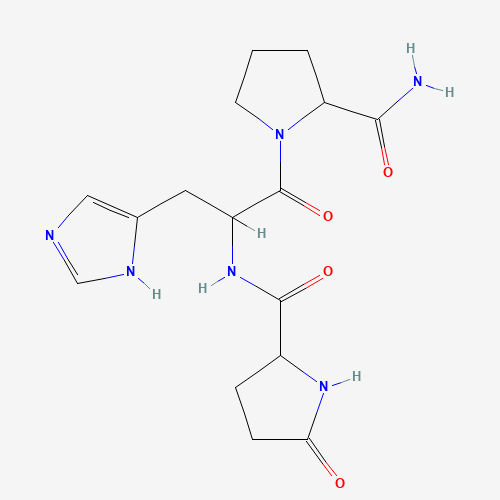
| SMILES |
C1CC(N(C1)C(=O)C(CC2=CN=CN2)NC(=O)C3CCC(=O)N3)C(=O)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

