| Pharmaceutical Information |
| Drug Name |
Technetium tc-99m oxidronate |
| Drug ID |
BADD_D02130 |
| Description |
Technetium Tc-99m oxidronate, also known as 99mTc-methylene diphosphonate, is a radiopharmaceutical agent. A radiopharmaceutical is defined as a medicinal formulation containing radioisotopes that are used in major clinical areas for diagnosis and/or therapy.[L1137] The radiopharmaceuticals based on technetium-99m are widely used for diagnostic purposes because 99mTc has a versatile chemistry which allows it to produce an extense variety of complexes with specific characteristics.[L1138] These complexes are formed by the binding of 99mTc to metal atoms of an organic molecule. The group oxidronate falls into the category of diphosphonates whose structure allows them to bind to calcium.[L1139] Thus, technetium Tc-99m oxidronate is a powerful detection tool for abnormal osteogenesis by skeletal scintigraphy.[L1140] It was developed by Mallinkrodt nuclear and FDA approved on February 18, 1981. |
| Indications and Usage |
Technetium Tc-99m oxidronate is indicated in adult and pediatric patients to be used in skeletal imaging for diagnosis of areas that can present altered osteogenesis. When administered intravenously, it is able to generate a clear image of the bones which allows the physician to diagnose any bone problem.[L1141] It is important to point out that this drug has to be manipulated only under the service of a nuclear specialist.[L1140] The approved indications for a bone scan are 1) visualization of tumor metastasis in bone, 2) osteomyelitis, 3) fracture, 4) stress fracture, 5) avascular necrosis, 6) osteoporosis and 7) prosthetic joint evaluation. From all the major indications, the detection of a metastatic disease is the most common because it presents a 95% of sensitivity and lesion detection can be done 6 months earlier than with X-ray studies.[L1139] |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09139
|
| KEGG ID |
Not Available
|
| MeSH ID |
C024226
|
| PubChem ID |
123801
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
MG4KI49HHJ
|
| Synonyms |
technetium Tc 99m hydroxymethylene diphosphonate | Tc-99m-oxidronate | Tc-99m-HMDP | Tc-99m hydroxymethylene diphosphonate | Tc-99m-HDP | technetium osteoscan-HDP | technetium salt (hydroxymethylene)bis(phosphonic acid) | technetium HMDP | Osteoscan |
|
| Chemical Information |
| Molecular Formula |
CH6O7P2Tc |
| CAS Registry Number |
72945-61-0 |
| SMILES |
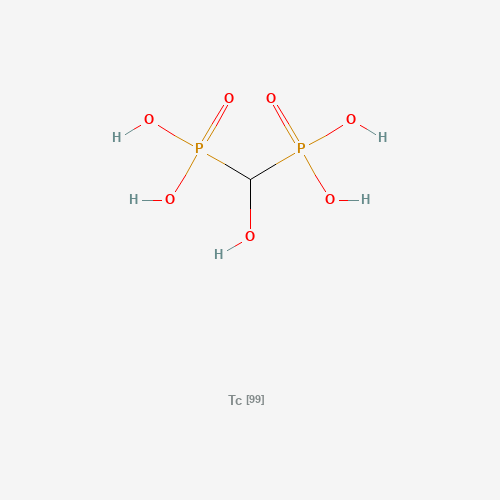
C(O)(P(=O)(O)O)P(=O)(O)O.[Tc] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Hypersensitivity | 10.01.03.003 | - | - | | | Nausea | 07.01.07.001 | - | - | | | Vomiting | 07.01.07.003 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

