| Pharmaceutical Information |
| Drug Name |
Rosuvastatin calcium |
| Drug ID |
BADD_D01972 |
| Description |
Rosuvastatin, also known as the brand name product Crestor, is a lipid-lowering drug that belongs to the statin class of medications, which are used to lower the risk of cardiovascular disease and manage elevated lipid levels by inhibiting the endogenous production of cholesterol in the liver. More specifically, statin medications competitively inhibit the enzyme hydroxymethylglutaryl-coenzyme A (HMG-CoA) Reductase,[A181421] which catalyzes the conversion of HMG-CoA to mevalonic acid and is the third step in a sequence of metabolic reactions involved in the production of several compounds involved in lipid metabolism and transport including cholesterol, low-density lipoprotein (LDL) (sometimes referred to as "bad cholesterol"), and very low-density lipoprotein (VLDL). Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD, such as those with Type 2 Diabetes. The clear evidence of the benefit of statin use coupled with very minimal side effects or long term effects has resulted in this class becoming one of the most widely prescribed medications in North America.[A181087, A181406] |
| Indications and Usage |
The FDA monograph states that rosuvastatin is indicated as an adjunct to diet in the treatment of triglyceridemia, Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia), and Homozygous Familial Hypercholesterolemia.[F4649]
The Health Canada monograph for rosuvastatin further specifies that rosuvastatin is indicated for the reduction of elevated total cholesterol (Total-C), LDL-C, ApoB, the Total-C/HDL-C ratio and triglycerides (TG) and for increasing HDL-C in hyperlipidemic and dyslipidemic conditions when response to diet and exercise alone has been inadequate. It is also indicated for the prevention of major cardiovascular events (including risk of myocardial infarction, nonfatal stroke, and coronary artery revascularization) in adult patients without documented history of cardiovascular or cerebrovascular events, but with at least two conventional risk factors for cardiovascular disease.[F4652]
Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.[A181087, A181406] |
| Marketing Status |
approved |
| ATC Code |
C10AA07 |
| DrugBank ID |
DB01098
|
| KEGG ID |
D01915
|
| MeSH ID |
D000068718
|
| PubChem ID |
5282455
|
| TTD Drug ID |
D0JE2E
|
| NDC Product Code |
65727-028; 66039-834; 13668-180; 27808-155; 42677-302; 43547-592; 50090-5970; 50090-6302; 51655-987; 0310-0752; 60687-256; 63187-869; 65862-294; 70518-2141; 70934-244; 70934-275; 71610-078; 71610-233; 72189-106; 82009-020; 55488-0320; 65372-1176; 65862-304; 70232-0004; 71492-100; 13668-179; 43353-352; 47335-583; 50090-4738; 51407-155; 57237-170; 0310-0755; 65862-293; 68788-8384; 70377-009; 70934-245; 71205-077; 71205-475; 71209-045; 71335-1103; 71335-2055; 71610-196; 71610-216; 72205-027; 82009-019; 0904-6781; 54921-759; 73680-0015; 27808-156; 50090-5188; 50090-5745; 50090-6524; 51407-154; 51655-756; 0310-0751; 63187-865; 68071-2634; 68071-2760; 68071-5258; 68462-263; 69539-009; 70518-1311; 70518-2542; 71205-008; 71335-0302; 72205-002; 0310-7560; 67877-441; 68788-7086; 70377-006; 70377-007; 70518-3687; 71205-099; 71209-046; 71335-1330; 71610-234; 72205-003; 0904-6780; 50923-0490; 54921-757; 69218-0100; 69218-0600; 16714-991; 43547-591; 47335-582; 47335-584; 50090-5468; 50090-6522; 55700-998; 0310-7570; 60687-245; 63629-7158; 63629-8124; 70377-008; 70518-2774; 71335-1440; 71335-2035; 81288-751; 82009-017; 54921-754; 65862-296; 65977-0077; 27808-157; 50090-5770; 51655-235; 57237-169; 0310-7590; 60687-234; 67877-439; 68462-264; 68788-8135; 70518-0484; 71205-052; 71205-390; 71335-1890; 72205-005; 14501-0002; 54921-752; 59285-005; 66039-914; 82608-005; 42677-301; 50090-6519; 57237-171; 63629-7157; 68462-261; 70518-2055; 71205-279; 71205-355; 71335-1017; 71610-187; 72205-004; 0904-6779; 49187-0200; 54921-751; 62512-0062; 66039-955; 68578-0011; 13668-182; 50090-2451; 50090-6422; 51407-153; 0310-0754; 70518-2090; 70518-3639; 70518-3651; 70518-3653; 71205-820; 71209-044; 71335-1741; 71335-1889; 71335-1939; 71610-232; 55111-863; 72640-013; 16714-988; 16714-989; 43353-332; 43547-593; 47335-585; 50090-5110; 51407-156; 55154-4163; 55700-839; 0310-7580; 63187-860; 65862-295; 68788-7612; 68788-7947; 68788-8368; 69539-008; 69539-010; 69539-160; 71205-176; 71209-043; 71335-1733; 71335-9640; 71610-133; 81288-755; 0904-6778; 13668-181; 16714-990; 27808-158; 43353-339; 50090-2447; 51655-256; 51655-309; 51655-996; 55700-885; 57237-168; 63187-864; 67877-440; 67877-442; 68071-2637; 68071-5225; 68462-262; 68788-8428; 70518-0375; 70518-0987; 70518-1819; 71205-078; 71335-0905; 71335-1098; 71610-215; 82009-018; 54921-755; 54921-756; 54921-758; 65015-752; 75945-030; 79677-000; 43547-594; 50090-5189; 50090-6077; 51655-062; 51655-937; 55154-8099 |
| UNII |
83MVU38M7Q
|
| Synonyms |
Rosuvastatin Calcium | Calcium, Rosuvastatin | Crestor | Rosuvastatin | ZD4522 | ZD 4522 |
|
| Chemical Information |
| Molecular Formula |
C44H54CaF2N6O12S2 |
| CAS Registry Number |
147098-20-2 |
| SMILES |
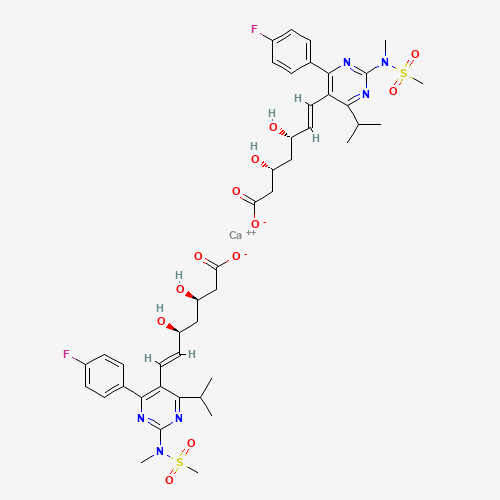
CC(C)C1=NC(=NC(=C1C=CC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)N(C)S(=O)(=O)C.CC(C)C1=N
C(=NC(=C1C=CC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)N(C)S(=O)(=O)C.[Ca+2] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

