| Pharmaceutical Information |
| Drug Name |
Regorafenib |
| Drug ID |
BADD_D01922 |
| Description |
Regorafenib is an orally-administered inhibitor of multiple kinases. It is used for the treatment of metastatic colorectal cancer, advanced gastrointestinal stromal tumours, and hepatocellular carcinoma. FDA approved on September 27, 2012. Approved use of Regorafenib was expanded to treat Hepatocellular Carcinoma in April 2017. |
| Indications and Usage |
Regorafenib is indicated for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy. Regorafenib is also indicated for the treatment of patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumour (GIST) who have been previously treated with imatinib mesylate and sunitinib malate. Regorafenib is also indicated for the treatment of patients with hepatocellular carcinoma (HCC) previously treated with sorafenib.[L16835] |
| Marketing Status |
approved |
| ATC Code |
L01EX05 |
| DrugBank ID |
DB08896
|
| KEGG ID |
D10138
|
| MeSH ID |
C559147
|
| PubChem ID |
11167602
|
| TTD Drug ID |
D09GDD
|
| NDC Product Code |
50419-171; 54893-0033; 12527-0171; 63415-0522 |
| UNII |
MGN125FS9D
|
| Synonyms |
regorafenib | 4-(4-(((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)amino)-3-fluorophenoxy)-n-methylpyridine-2-carboxamide | Stivarga | BAY 73-4506 | BAY73-4506 | BAY-73-4506 |
|
| Chemical Information |
| Molecular Formula |
C21H15ClF4N4O3 |
| CAS Registry Number |
755037-03-7 |
| SMILES |
CNC(=O)C1=NC=CC(=C1)OC2=CC(=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F)F |
| Chemical Structure |
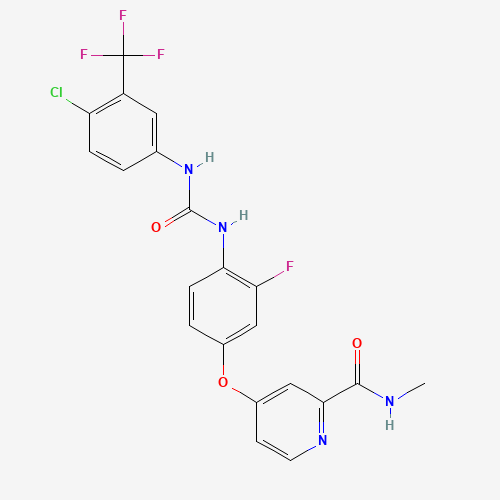
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Urinary fistula | 20.08.01.017 | 0.000112% | | |
|
|
|

