| Pharmaceutical Information |
| Drug Name |
Pyridostigmine |
| Drug ID |
BADD_D01881 |
| Description |
Myasthenia gravis is an autoimmune disease involving dysfunction at the neuromuscular junction, most commonly due to autoantibodies directed against the acetylcholine receptor (AChR), which results in muscle tone loss, muscle weakness, and fatigue.[A231004] Acetylcholinesterase inhibitors have been the symptomatic treatment of choice in myasthenia gravis since the 1930s with the early use of [physostigmine] and [neostigmine]. By inhibiting the breakdown of acetylcholine in the neuromuscular junction, they increase signalling and relieve symptoms.[A231004, L32408, L32413] Pyridostigmine is the current drug of choice, with superior pharmacokinetics and reduced side effects compared to [neostigmine].[L32408, L32413] In addition to treating myasthenia gravis, pyridostigmine is used to reverse neuromuscular blocks, relieve symptoms in congenital myasthenic syndromes, and protect against certain nerve agents, notably during the Gulf War.[A231009, A231014, L32413, L32418]
Pyridostigmine was granted initial FDA approval on April 6, 1955, as an oral tablet. Possible dose forms have been expanded to include extended-release tablets, syrups, and injections, marketed under various brand and generic names.[L32408, L32413] |
| Indications and Usage |
Pyridostigmine is indicated for the treatment of myasthenia gravis.[L32408] When administered intravenously, it is indicated for the reversal or antagonism of the neuromuscular blocking effects of nondepolarizing muscle relaxants.[L32413]
Pyridostigmine has also been used as a prophylactic agent against irreversible organophosphorus acetylcholinesterase inhibitors, primarily in a military capacity.[L32418] |
| Marketing Status |
approved; investigational |
| ATC Code |
N07AA02 |
| DrugBank ID |
DB00545
|
| KEGG ID |
D00487
|
| MeSH ID |
D011729
|
| PubChem ID |
4991
|
| TTD Drug ID |
D0O2WB
|
| NDC Product Code |
Not Available |
| UNII |
19QM69HH21
|
| Synonyms |
Pyridostigmine Bromide | Bromide, Pyridostigmine | Pyridostigmine | Mestinon |
|
| Chemical Information |
| Molecular Formula |
C9H13N2O2+ |
| CAS Registry Number |
155-97-5 |
| SMILES |
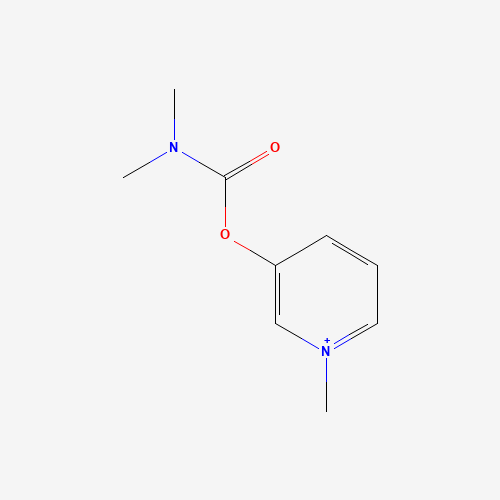
C[N+]1=CC=CC(=C1)OC(=O)N(C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

