| Pharmaceutical Information |
| Drug Name |
Prucalopride |
| Drug ID |
BADD_D01874 |
| Description |
Prucalopride is a dihydrobenzofurancarboxamide derivative from the benzofurane family that selectively stimulates 5-HT4 receptors and thus, it presents enterokinetic properties.[A37348] The high selectivity of prucalopride allowed further development as it prevented the cardiac adverse reactions observed due to non-target effects of precedent therapies.[A40254] Prucalopride was developed by Shire Development LLC and approved for use in Europe in 2009,[A40250] in Canada on December 7, 2011 and by the FDA on December 17, 2018.[L4880] |
| Indications and Usage |
Prucalopride is indicated for the treatment of chronic idiopathic constipation (CIC) in adults.[L4882]
CIC is one of the most common chronic functional gastrointestinal disorders worldwide. The diagnosis of this agent is very hard and it can be confirmed if the patient experience at least two of the following:
-Straining during more than 25% of the bowel movements.
-Lumpy or hard stools in 25% of the bowel movements.
-Sensation of incomplete evacuation in more than 25% of all bowel movements.
-Sensation of anorectal blockage or obstruction in more than 25% of the bowel movements.
-Manual maneuvers required in more than 25% of the bowel movements.
-Fewer than 3 bowel movements per week.[L4883] |
| Marketing Status |
approved |
| ATC Code |
A06AX05 |
| DrugBank ID |
DB06480
|
| KEGG ID |
D09205
|
| MeSH ID |
C406662
|
| PubChem ID |
3052762
|
| TTD Drug ID |
D04QSJ
|
| NDC Product Code |
54092-546; 54092-547 |
| UNII |
0A09IUW5TP
|
| Synonyms |
prucalopride | 4-amino-5-chloro-N-(1-(3-methoxypropyl)-4-piperidinyl)-2,3-dihydro-1-benzofuran-7-carboxamide | motegrity | resotran | resotrans | R 093877 | R093877 | Resolor |
|
| Chemical Information |
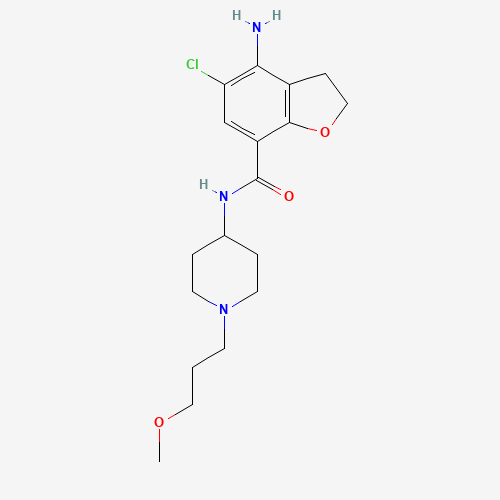
| Molecular Formula |
C18H26ClN3O3 |
| CAS Registry Number |
179474-81-8 |
| SMILES |
COCCCN1CCC(CC1)NC(=O)C2=CC(=C(C3=C2OCC3)N)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

