| Pharmaceutical Information |
| Drug Name |
Prednicarbate |
| Drug ID |
BADD_D01830 |
| Description |
Prednicarbate is a relatively new topical corticosteroid drug that displays a similar potency as [hydrocortisone]. It is used in the treatment of inflammatory skin diseases, such as atopic dermatitis. It has a favorable benefit-risk ratio, with an inflammatory action similar to that of a medium potency corticosteroid, but with a low potential to cause skin atrophy. The anti-inflammation action of corticosteroids is associated with the inhibition of the interleukin 1-alpha cytokine within keratinocytes. IL-1a is also found in fibroblasts, where it is responsible for proliferation, collagenase induction and IL-6 synthesis, which are related to skin thickness. |
| Indications and Usage |
For the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. |
| Marketing Status |
approved; investigational |
| ATC Code |
D07AC18 |
| DrugBank ID |
DB01130
|
| KEGG ID |
D05601
|
| MeSH ID |
C035287
|
| PubChem ID |
52421
|
| TTD Drug ID |
D09IEE
|
| NDC Product Code |
63592-2140; 65089-0032; 52128-168; 0168-0410; 24002-0006 |
| UNII |
V901LV1K7D
|
| Synonyms |
prednicarbate | prednisolone-17-ethylcarbonate-21-propionate | Peitel | HOE 777 | HOE-777 | Batmen | Dermatop |
|
| Chemical Information |
| Molecular Formula |
C27H36O8 |
| CAS Registry Number |
73771-04-7 |
| SMILES |
CCC(=O)OCC(=O)C1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)OC(=O)OCC |
| Chemical Structure |
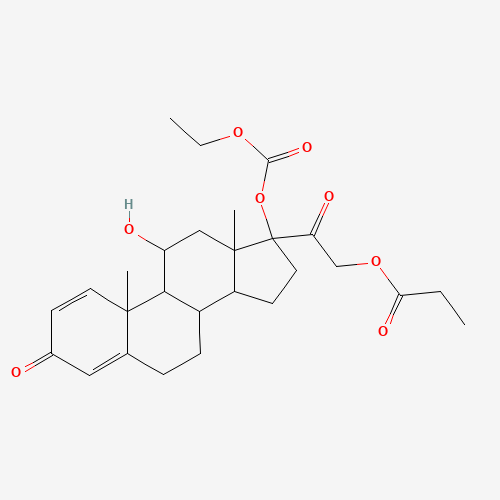
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

