| Pharmaceutical Information |
| Drug Name |
Phenylephrine |
| Drug ID |
BADD_D01762 |
| Description |
Phenylephrine is an alpha-1 adrenergic receptor agonist used to treat hypotension,[L9416,L9410] dilate the pupil,[L9413] and induce local vasoconstriction.[A187370] The action of phenylephrine, or neo-synephrine, was first described in literature in the 1930s.[A187376]
Phenylephrine was granted FDA approval in 1939.[L9413] |
| Indications and Usage |
Phenylephrine injections are indicated to treat hypotension caused by shock or anesthesia,[L9416, L9410] an ophthalmic formulation is indicated to dilate pupils[L9413] and induce vasoconstriction, an intranasal formulation is used to treat congestion, and a topical formulation is used to treat hemorrhoids.[A187370] Off-label uses include situations that require local blood flow restriction such as the treatment of priapism.[A187370] |
| Marketing Status |
approved |
| ATC Code |
C01CA06; R01AA04; R01AB01; R01BA03; S01FB01; S01GA05 |
| DrugBank ID |
DB00388
|
| KEGG ID |
D08365
|
| MeSH ID |
D010656
|
| PubChem ID |
6041
|
| TTD Drug ID |
D0O6IU
|
| NDC Product Code |
57218-710 |
| UNII |
1WS297W6MV
|
| Synonyms |
Phenylephrine | (R)-3-Hydroxy-alpha-((methylamino)methyl)benzenemethanol | Metasympatol | Mezaton | Phenylephrine Hydrochloride | Phenylephrine Tannate | Tannate, Phenylephrine | Metaoxedrin | Neo-Synephrine | Neo Synephrine | Neosynephrine |
|
| Chemical Information |
| Molecular Formula |
C9H13NO2 |
| CAS Registry Number |
59-42-7 |
| SMILES |
CNCC(C1=CC(=CC=C1)O)O |
| Chemical Structure |
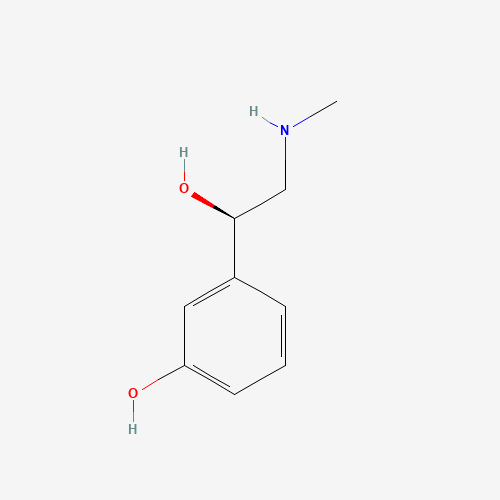
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

