| Pharmaceutical Information |
| Drug Name |
Lesinurad |
| Drug ID |
BADD_D01256 |
| Description |
Lesinurad is an oral uric acid transporter 1 (URAT1) inhibitor indicated for the treatment of hyperuricemia associated with gout. It reduces serum uric acid concentration through the inhibition of URAT1, an enzyme responsible for reuptake of uric acid from the renal tubule, and OAT4, another uric acid transporter associated with diuretic-induced hyperuricemia.
Marketed as the product Zurampic, it is indicated for use in combination with a xanthine oxidase inhibitor for the treatment of hyperuricemia associated with gout in
patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone. In August 2017, a combination oral therapy consisting of lesinurad and [DB00437] was FDA-approved under the brand name Duzallo indicated for the treatment of gout-related hyperuricemia in patients with uncontrolled gout. |
| Indications and Usage |
For use, in combination with a xanthine oxidase inhibitor, for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a xanthine oxidase inhibitor alone. |
| Marketing Status |
approved; investigational |
| ATC Code |
M04AB05 |
| DrugBank ID |
DB11560
|
| KEGG ID |
D09921
|
| MeSH ID |
C000593471
|
| PubChem ID |
53465279
|
| TTD Drug ID |
D0C3SW
|
| NDC Product Code |
72640-006 |
| UNII |
09ERP08I3W
|
| Synonyms |
lesinurad | ((5-bromo-4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazol-3-yl)sulfanyl)acetic acid | RDEA594 | Zurampic |
|
| Chemical Information |
| Molecular Formula |
C17H14BrN3O2S |
| CAS Registry Number |
878672-00-5 |
| SMILES |
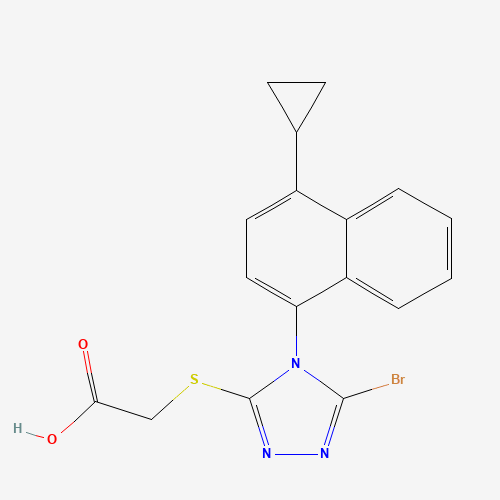
C1CC1C2=CC=C(C3=CC=CC=C23)N4C(=NN=C4Br)SCC(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

