| Pharmaceutical Information |
| Drug Name |
Ioversol |
| Drug ID |
BADD_D01186 |
| Description |
Ioversol is classified as an _organoiodine_ compound and is used as a contrast dye in diagnostic procedures. It contains high levels of iodine in addition to various hydrophilic groups. |
| Indications and Usage |
Optiray 350 is indicated in adults for peripheral and coronary arteriography and left ventriculography. Optiray 350 is also indicated for contrast enhanced computed tomographic imaging of the head and body, intravenous excretory urography, intravenous digital subtraction angiography and venography. Optiray 350 is indicated in children for angiocardiography.
Optiray 320 is indicated in adults for angiography throughout the cardiovascular system. The uses include cerebral, coronary, peripheral, visceral and renal arteriography, venography, aortography, and left ventriculography. Optiray 320 is also indicated for contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography.
Optiray 320 is indicated in children for angiocardiography, contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography.
Optiray 300 is indicated for cerebral angiography and peripheral arteriography. Optiray 300 is also indicated for contrast enhanced computed tomographic imaging of the head and body, venography, and intravenous excretory urography.
Optiray 240 is indicated for cerebral angiography and venography. Optiray 240 is also indicated for contrast enhanced computed tomographic imaging of the head and body and intravenous excretory urography. |
| Marketing Status |
approved |
| ATC Code |
V08AB07 |
| DrugBank ID |
DB09134
|
| KEGG ID |
D01555
|
| MeSH ID |
C054871
|
| PubChem ID |
3741
|
| TTD Drug ID |
D0U1ZD
|
| NDC Product Code |
0019-1323; 0019-1332; 0406-7888; 0019-1333; 57884-0015; 67684-8777 |
| UNII |
N3RIB7X24K
|
| Synonyms |
ioversol | Optiray 320 | Optiray | Optiray 300 |
|
| Chemical Information |
| Molecular Formula |
C18H24I3N3O9 |
| CAS Registry Number |
87771-40-2 |
| SMILES |
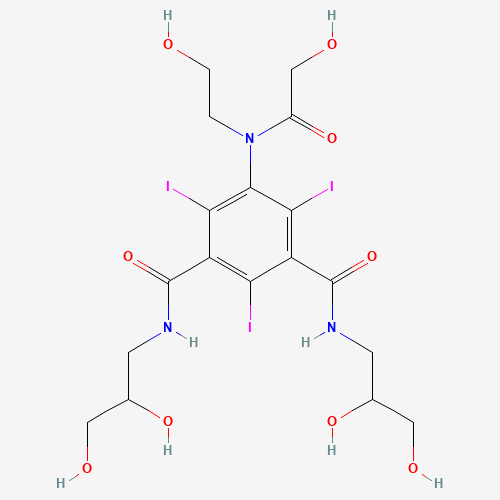
C(CO)N(C1=C(C(=C(C(=C1I)C(=O)NCC(CO)O)I)C(=O)NCC(CO)O)I)C(=O)CO |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

