| Pharmaceutical Information |
| Drug Name |
Iodinated i-131 serum albumin |
| Drug ID |
BADD_D01175 |
| Description |
Iodinated I-131 serum albumin is a radiopharmaceutical agent used for several diagnostic purposes, including the determination of body fluid volumes and the imaging of certain tissues.[L12783] |
| Indications and Usage |
Iodinated I-131 albumin is indicated for use in determinations of total blood and plasma volumes, cardiac output, cardiac and pulmonary blood volumes and circulation times, and in protein turnover studies, heart and great vessel delineation, localization of the placenta, and localization of cerebral neoplasms.[L12783] |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09430
|
| KEGG ID |
Not Available
|
| MeSH ID |
D012711
|
| PubChem ID |
10176205
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
Not Available
|
| Synonyms |
Serum Albumin, Radio-Iodinated | Albumin, Radio-Iodinated Serum | Radio-Iodinated Serum Albumin | Serum Albumin, Radio Iodinated | Serum Albumin, Radioiodinated | Albumin, Radioiodinated Serum | Radioiodinated Serum Albumin | (131)I-Macroaggregated Albumin | (131)I-MAA |
|
| Chemical Information |
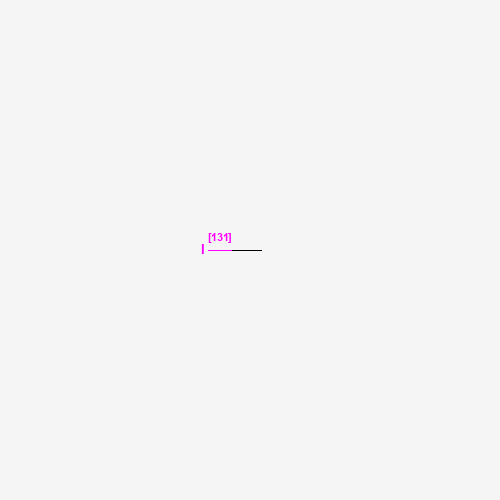
| Molecular Formula |
CH3I |
| CAS Registry Number |
Not Available |
| SMILES |
CI |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Hypersensitivity | 10.01.03.003 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

