| Pharmaceutical Information |
| Drug Name |
Fosfomycin tromethamine |
| Drug ID |
BADD_D00966 |
| Description |
Fosfomycin was discovered in 1969 by scientists at the Spanish Penicillin and Antibiotics Company and is produced by _Streptomyces fradiae_.[A229338,A230348] It may also be produced synthetically and is commercially available as the disodium salt for intravenous administration and as the calcium or trometamol salt for oral administration.[A230348] In terms of chemical structure, fosfomycin is a phosphoenolpyruvate analog and contains a phosphonic group and an epoxide ring.[A230348]
Due to its ease of administration as a single 3-gram oral dose and desirable safety profile, fosfomycin has largely become a first-line therapeutic option for the treatment of uncomplicated urinary tract infections (UTIs) in females.[A230378] Despite being FDA approved only for urinary tract infections, fosfomycin actually has a broad spectrum of activity and is active against both gram-positive and gram-negative bacteria.[A230348] As such there is great interest in exploring the usefulness of fosfomycin for indications beyond the treatment of UTIs.[A230353,A230358] |
| Indications and Usage |
Fosfomycin is indicated for the treatment of uncomplicated cases of cystitis caused by susceptible strains of _Escherichia coli_ and _Enterococcus faecalis_.[L31818] Fosfomycin is not officially indicated for the treatment of pyelonephritis or perinephric abscess, although there have been reported cases of off-label usage in these situations.[L31818] |
| Marketing Status |
approved |
| ATC Code |
S02AA17; J01XX01 |
| DrugBank ID |
DB00828
|
| KEGG ID |
D00925
|
| MeSH ID |
D005578
|
| PubChem ID |
54331
|
| TTD Drug ID |
D01GYT
|
| NDC Product Code |
53069-1080; 50090-6130; 82036-4274; 0456-4300; 69037-0066; 63629-9470; 67877-749; 69097-579; 12836-0318 |
| UNII |
7FXW6U30GY
|
| Synonyms |
Fosfomycin | Phosphomycin | Phosphonomycin | Monuril | Fosfomycin Trometamol Salt | Fosfomycin Tromethamine | Tromethamine, Fosfomycin |
|
| Chemical Information |
| Molecular Formula |
C7H18NO7P |
| CAS Registry Number |
78964-85-9 |
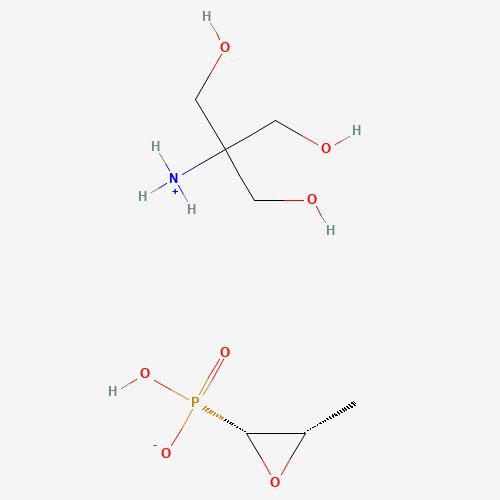
| SMILES |
CC1C(O1)P(=O)(O)[O-].C(C(CO)(CO)[NH3+])O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

