| Pharmaceutical Information |
| Drug Name |
Dimethyl fumarate |
| Drug ID |
BADD_D00680 |
| Description |
Dimethyl fumarate is an anti-inflammatory. It is indicated for multiple sclerosis patients with relapsing forms and is also being investigated for the treatment of psoriasis. The mechanism of action of dimethyl fumarate in multiple sclerosis is not well understood. It is thought to involve dimethyl fumarate degradation to its active metabolite monomethyl fumarate (MMF) then MMF up-regulates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway that is activated in response to oxidative stress. Dimethyl fumarate is marketed under the brand name Tecfidera. |
| Indications and Usage |
Used in multiple sclerosis patients with relapsing forms. |
| Marketing Status |
approved; investigational |
| ATC Code |
L04AX07 |
| DrugBank ID |
DB08908
|
| KEGG ID |
D03846
|
| MeSH ID |
D000069462
|
| PubChem ID |
637568
|
| TTD Drug ID |
D0A7MY
|
| NDC Product Code |
69037-0012; 0093-9219; 70771-1531; 55111-975; 76055-0026; 51407-441; 49812-0255; 58159-013; 62207-003; 66039-901; 66577-043; 82920-027; 16729-416; 24979-128; 43598-429; 43598-430; 51407-442; 59651-083; 69539-042; 69539-043; 64406-005; 70710-1205; 54864-663; 55111-997; 59285-003; 62675-0393; 69667-1229; 16729-417; 31722-657; 0378-0396; 10920-598; 65015-844; 24979-127; 0378-0399; 64406-007; 67877-555; 69097-323; 70512-853; 70771-1532; 59651-061; 62512-0074; 59651-084; 62579-301; 43547-024; 50090-5288; 67877-556; 69238-1318; 69238-1319; 70512-852; 70710-1416; 70771-1530; 50379-0013; 31722-680; 43547-025; 64406-006; 69097-322; 69097-552; 69238-1626; 69539-240; 82245-0105; 31722-658; 67877-557; 70710-1204 |
| UNII |
FO2303MNI2
|
| Synonyms |
Dimethyl Fumarate | Fumarate, Dimethyl | 2-butenedioic acid, (2E)-, dimethyl ester | Dimethylfumarate | BG 00012 | 00012, BG | BG00012 | BG-00012 | Tecfidera | FAG 201 | 201, FAG | FAG201 | FAG-201 | Fumaderm | BG 12 compound | 12 compound, BG | compound, BG 12 | BG12 compound | compound, BG12 | BG-12 compound |
|
| Chemical Information |
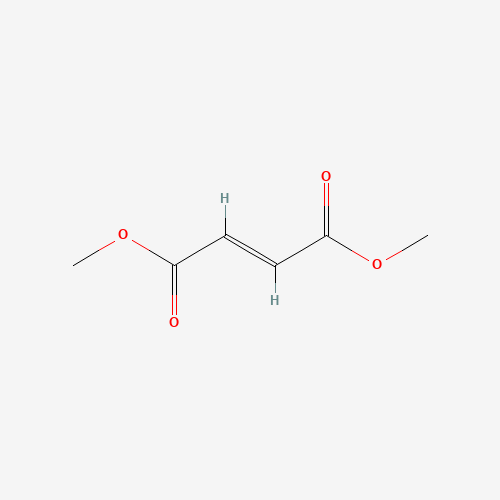
| Molecular Formula |
C6H8O4 |
| CAS Registry Number |
624-49-7 |
| SMILES |
COC(=O)C=CC(=O)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Vaccination failure | 08.06.01.069; 12.02.11.007 | 0.000880% | | - | | Vein collapse | 12.02.01.040; 24.03.02.039 | 0.000400% | | - |
|
|
|

