| Pharmaceutical Information |
| Drug Name |
Degarelix acetate |
| Drug ID |
BADD_D00600 |
| Description |
Degarelix is used for the treatment of advanced prostate cancer. Degarelix is a synthetic peptide derivative drug which binds to gonadotropin-releasing hormone (GnRH) receptors in the pituitary gland and blocks interaction with GnRH. This antagonism reduces luteinising hormone (LH) and follicle-stimulating hormone (FSH) which ultimately causes testosterone suppression. Reduction in testosterone is important in treating men with advanced prostate cancer. Chemically, it is a synthetic linear decapeptide amide with seven unnatural amino acids, five of which are D-amino acids. FDA approved on December 24, 2008. |
| Indications and Usage |
Degaralix is used for the management of advanced prostate cancer. |
| Marketing Status |
approved |
| ATC Code |
L02BX02 |
| DrugBank ID |
DB06699
|
| KEGG ID |
D08635; D09400
|
| MeSH ID |
C431566
|
| PubChem ID |
16186010
|
| TTD Drug ID |
D0Y7KH
|
| NDC Product Code |
14799-3003; 66529-0017; 66558-0194; 70155-022; 61662-0015 |
| UNII |
I18S89P20R
|
| Synonyms |
acetyl-2-naphthylalanyl-3-chlorophenylalanyl-1-oxohexadecyl-seryl-4-aminophenylalanyl(hydroorotyl)-4-aminophenylalanyl(carbamoyl)-leucyl-ILys-prolyl-alaninamide | uglypeptide1 | Ac-2Nal-4Cpa-3Pal-Ser-4Aph(hydroorotyl)-4Aph(carbamoyl)-Leu-ILys-pro-Ala-NH2 | Firmagon | degarelix | degarelix acetate | Gonax | FE 200486 | FE200486 | FE-200486 |
|
| Chemical Information |
| Molecular Formula |
C84H107ClN18O18 |
| CAS Registry Number |
934016-19-0 |
| SMILES |
CC(C)CC(C(=O)NC(CCCCNC(C)C)C(=O)N1CCCC1C(=O)NC(C)C(=O)N)NC(=O)C(CC2=CC=C(C=C2)NC
(=O)N)NC(=O)C(CC3=CC=C(C=C3)NC(=O)C4CC(=O)NC(=O)N4)NC(=O)C(CO)NC(=O)C(CC5=CN=CC=
C5)NC(=O)C(CC6=CC=C(C=C6)Cl)NC(=O)C(CC7=CC8=CC=CC=C8C=C7)NC(=O)C.CC(=O)O |
| Chemical Structure |
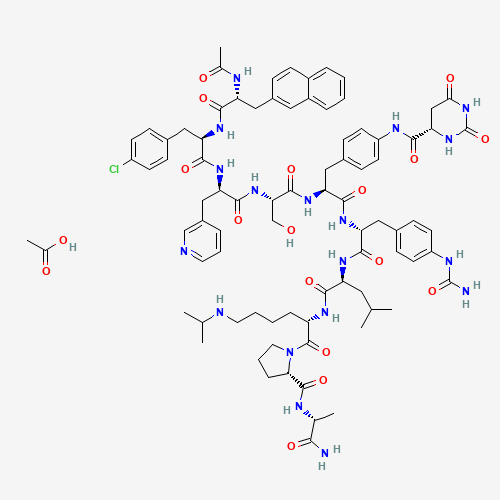
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

