| Pharmaceutical Information |
| Drug Name |
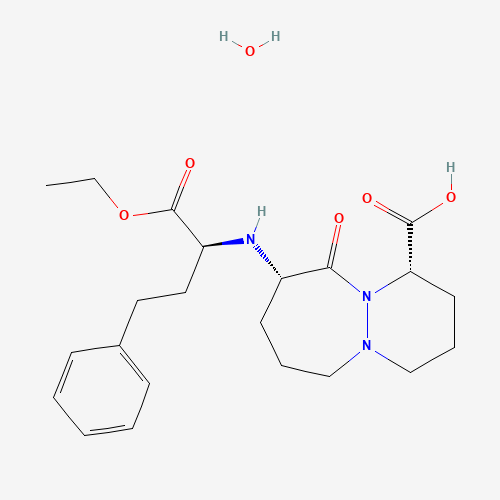
Cilazapril monohydrate |
| Drug ID |
BADD_D00465 |
| Description |
Cilazapril is an ACE inhibtor class drug used in the treatment of hypertension and heart failure. It belongs to the angiotensin-converting enzyme inhibitors (ACE inhibitors) class of drugs. It is a prodrug that is hydrolyzed after absorption to its main metabolite cilazaprilat. It is branded as Inhibace in Canada and other countries, Vascace and Dynorm in a number of European countries, among many other names. None of these varieties are available in the United States. |
| Indications and Usage |
Cilazapril is an ACE inhibtor class drug used in the treatment of hypertension and heart failure. |
| Marketing Status |
approved |
| ATC Code |
C09AA08 |
| DrugBank ID |
DB01340
|
| KEGG ID |
D07699; D01069
|
| MeSH ID |
D017315
|
| PubChem ID |
56329
|
| TTD Drug ID |
D04GKO
|
| NDC Product Code |
Not Available |
| UNII |
19KW7PI29F
|
| Synonyms |
Cilazapril | Cilazapril Monohydrobromide | Cilazapril, Anhydrous | Cilazapril Anhydrous | Cilazapril Monohydrate | Ro 31-2848 | Ro 31 2848 | Ro 312848 | Ro-31-2848 | Ro312848 | Cilazapril Hydrate | Cilazapril, (S*)-Isomer | Inhibace |
|
| Chemical Information |
| Molecular Formula |
C22H33N3O6 |
| CAS Registry Number |
92077-78-6 |
| SMILES |
CCOC(=O)C(CCC1=CC=CC=C1)NC2CCCN3CCCC(N3C2=O)C(=O)O.O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

