| Pharmaceutical Information |
| Drug Name |
Chromium |
| Drug ID |
BADD_D00459 |
| Description |
Chromium is a transition element with the chemical symbol Cr and atomic number 24 that belongs to Group 6 of the periodic table. It is used in various chemical, industrial and manufacturing applications such as wood preservation and metallurgy. The uses of chromium compounds depend on the valency of chromium, where trivalent Cr (III) compounds are used for dietary Cr supplementation and hexavalent Cr (VI) compounds are used as corrosion inhibitors in commercial settings and are known to be human carcinogens [L1982]. Humans can be exposed to chromium via ingestion, inhalation, and dermal or ocular exposure [L1983]. Trivalent chromium (Cr(III)) ion is considered to be an essential dietary trace element as it is involved in metabolism of blood glucose, regulation of insulin resistance and metabolism of lipids. Clinical trials and other studies suggest the evidence of chromium intake improving glucose tolerance in patients with Type I and II diabetes, however its clinical application in the standard management of type II diabetes mellitus is not established. Chromium deficiency has been associated with a diabetic-like state, impaired growth, decreased fertility and increased risk of cardiovascular diseases [A32343, A32351, L1982].
According to the National Institute of Health, the daily dietary reference intake (DRI) of chromium for adult male and non-pregnant female are 35 μg and 25 μg, respectively [L1986]. Chromium picolinate capsules may be used as nutritional adjuvant in patients with or at risk of type 2 diabetes mellitus (T2DM) to improve blood sugar metabolism and stabilize the levels of serum cholesterol. Chromium chloride is available as an intravenous injection for use as a supplement to intravenous solutions given for total parenteral nutrition (TPN) [FDA Label]. |
| Indications and Usage |
Indicated for use as a supplement to intravenous solutions given for total parenteral nutrition (TPN), to maintain chromium serum levels and to prevent depletion of endogenous stores and subsequent deficiency symptoms [FDA Label]. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB11136
|
| KEGG ID |
Not Available
|
| MeSH ID |
D002857
|
| PubChem ID |
23976
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
0R0008Q3JB
|
| Synonyms |
Chromium |
|
| Chemical Information |
| Molecular Formula |
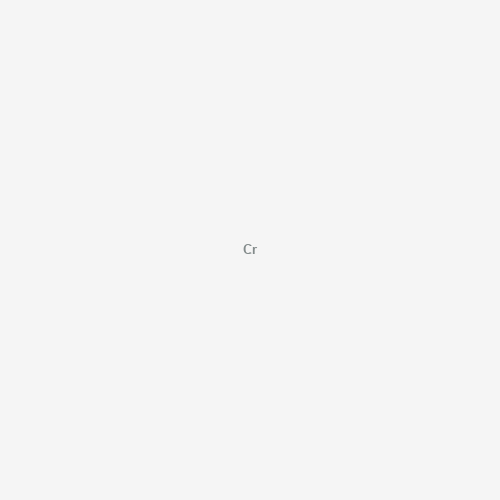
Cr |
| CAS Registry Number |
7440-47-3 |
| SMILES |
[Cr] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

