| Pharmaceutical Information |
| Drug Name |
Chlorpropamide |
| Drug ID |
BADD_D00447 |
| Description |
Chlorpropamide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM). It belongs to the sulfonylurea class of insulin secretagogues, which act by stimulating β cells of the pancreas to release insulin. Sulfonylureas increase both basal insulin secretion and meal-stimulated insulin release. Medications in this class differ in their dose, rate of absorption, duration of action, route of elimination and binding site on their target pancreatic β cell receptor. Sulfonylureas also increase peripheral glucose utilization, decrease hepatic gluconeogenesis and may increase the number and sensitivity of insulin receptors. Sulfonylureas are associated with weight gain, though less so than insulin. Due to their mechanism of action, sulfonylureas may cause hypoglycemia and require consistent food intake to decrease this risk. The risk of hypoglycemia is increased in elderly, debilitated and malnourished individuals. Chlorpropamide is not recommended for the treatment of NIDDM as it increases blood pressure and the risk of retinopathy (UKPDS-33). Up to 80% of the single oral dose of chlorpropramide is metabolized, likely in the liver; 80-90% of the dose is excreted in urine as unchanged drug and metabolites. Renal and hepatic dysfunction may increase the risk of hypoglycemia. |
| Indications and Usage |
For treatment of NIDDM in conjunction with diet and exercise. |
| Marketing Status |
approved; investigational |
| ATC Code |
A10BB02 |
| DrugBank ID |
DB00672
|
| KEGG ID |
D00271
|
| MeSH ID |
D002747
|
| PubChem ID |
2727
|
| TTD Drug ID |
D00BCP
|
| NDC Product Code |
52537-004 |
| UNII |
WTM2C3IL2X
|
| Synonyms |
Chlorpropamide | Clorpropamid | Diabinese | Insogen | Apo-Chlorpropamide | Glucamide | Meldian |
|
| Chemical Information |
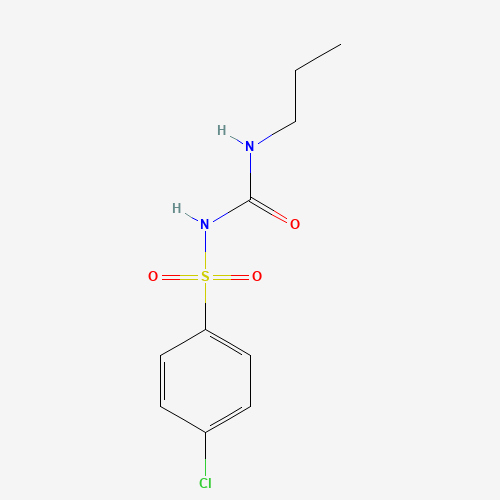
| Molecular Formula |
C10H13ClN2O3S |
| CAS Registry Number |
94-20-2 |
| SMILES |
CCCNC(=O)NS(=O)(=O)C1=CC=C(C=C1)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

