| Pharmaceutical Information |
| Drug Name |
Carbinoxamine |
| Drug ID |
BADD_D00361 |
| Description |
Carbinoxamine is a first generation antihistamine that competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the negative symptoms brought on by histamine HA-receptor binding. The product label for carbinoxamine as an over the counter cough and cold medicine is being modified to state "do not use" in children under 4 years of age in order to prevent and reduce misuse, as many unapproved carbinoxamine-containing preparations contained inappropriate labeling, which promoted unapproved uses (including management of congestion, cough, the common cold, and the use in children under 2 years of age), which can potentially cause serious health risks. |
| Indications and Usage |
For symptomatic relief of seasonal and perennial allergic rhinitis and vasomotor rhinitis, as well as allergic conjunctivitis caused by foods and inhaled allergens. Also for the relief of allergic reactions to blood or plasma, and the symptomatic management of mild, uncomplicated allergic skin manifestations of urticaria and angioedema. |
| Marketing Status |
approved |
| ATC Code |
R06AA08 |
| DrugBank ID |
DB00748
|
| KEGG ID |
D07617
|
| MeSH ID |
C004649
|
| PubChem ID |
2564
|
| TTD Drug ID |
D00FGV
|
| NDC Product Code |
Not Available |
| UNII |
982A7M02H5
|
| Synonyms |
carbinoxamine | Histex PD | Histex I-E | carbinoxamine maleate | Histex CT |
|
| Chemical Information |
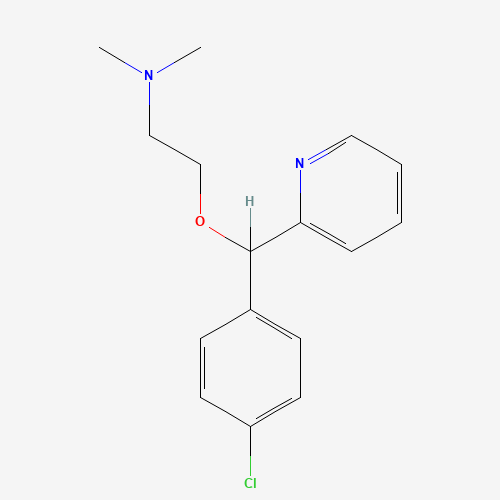
| Molecular Formula |
C16H19ClN2O |
| CAS Registry Number |
486-16-8 |
| SMILES |
CN(C)CCOC(C1=CC=C(C=C1)Cl)C2=CC=CC=N2 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

