| Pharmaceutical Information |
| Drug Name |
Bromocriptine mesylate |
| Drug ID |
BADD_D00305 |
| Description |
Bromocriptine mesylate is a semisynthetic ergot alkaloid derivative with potent dopaminergic activity. It is indicated for the management of signs and symptoms of Parkinsonian Syndrome. Bromocriptine also inhibits prolactin secretion and may be used to treat dysfunctions associated with hyperprolactinemia. It also causes sustained suppression of somatotropin (growth hormone) secretion in some patients with acromegaly. Bromocriptine has been associated with pulmonary fibrosis. |
| Indications and Usage |
For the treatment of galactorrhea due to hyperprolactinemia, prolactin-dependent menstrual disorders and infertility, prolactin-secreting adenomas, prolactin-dependent male hypogonadism, as adjunct therapy to surgery or radiotherapy for acromegaly or as monotherapy is special cases, as monotherapy in early Parksinsonian Syndrome or as an adjunct with levodopa in advanced cases with motor complications. Bromocriptine has also been used off-label to treat restless legs syndrome and neuroleptic malignant syndrome. |
| Marketing Status |
approved; investigational |
| ATC Code |
G02CB01; N04BC01 |
| DrugBank ID |
DB01200
|
| KEGG ID |
D00780
|
| MeSH ID |
D001971
|
| PubChem ID |
31100
|
| TTD Drug ID |
D06YFA
|
| NDC Product Code |
0574-0106; 55486-1561; 30698-201; 0781-5325; 0378-7096; 68012-258; 60687-286; 65841-654; 73212-035; 51927-2274; 30698-202; 68382-110 |
| UNII |
FFP983J3OD
|
| Synonyms |
Bromocriptine | 2-Bromo-alpha-ergokryptine | 2 Bromo alpha ergokryptine | Bromocryptin | 2-Bromoergokryptine | 2 Bromoergokryptine | Bromocriptin | 2-Bromoergocryptine | 2 Bromoergocryptine | 2-Bromo-alpha-ergocryptine | 2 Bromo alpha ergocryptine | CB-154 | CB 154 | CB154 | Parlodel | 2-Bromoergocryptine Mesylate | 2 Bromoergocryptine Mesylate | Mesylate, 2-Bromoergocryptine | Bromocriptine Mesylate | Mesylate, Bromocriptine | 2-Bromoergocryptine Methanesulfonate | 2 Bromoergocryptine Methanesulfonate | Methanesulfonate, 2-Bromoergocryptine |
|
| Chemical Information |
| Molecular Formula |
C33H44BrN5O8S |
| CAS Registry Number |
22260-51-1 |
| SMILES |
CC(C)CC1C(=O)N2CCCC2C3(N1C(=O)C(O3)(C(C)C)NC(=O)C4CN(C5CC6=C(NC7=CC=CC(=C67)C5=C
4)Br)C)O.CS(=O)(=O)O |
| Chemical Structure |
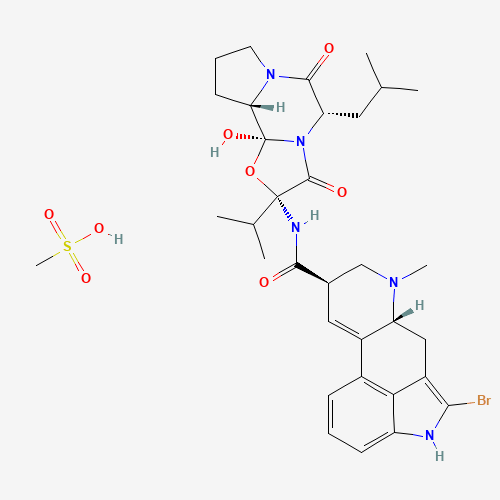
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Gambling disorder | 19.07.06.015 | - | - | - |
|
|
|

