| Pharmaceutical Information |
| Drug Name |
Belinostat |
| Drug ID |
BADD_D00225 |
| Description |
Belinostat is a novel agent that inhibits the enzyme histone deacetylase (HDAC) with a sulfonamide-hydroxamide structure. It was developed as an orphan drug to target hematological malignancies and solid tumors by TopoTarget. The safety and efficacy of belinostat is currently being evaluated for use in combination with traditional front-line therapies for the treatment of PTCL. Intravenous administration of the agent is available as Beleodaq as monotherapy and the dosing regimen involves a 21-day cycle. It was US-approved in July 2014 as a therapeutic agent for relapsed or refractory peripheral T-cell lymphoma. |
| Indications and Usage |
Belinostat is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) with manageable safety profile. It is a potential alternative therapy for patients who did not experience adequate response to first-line drugs for PTCL. It can be used in patients with baseline thrombocytopenia [A19161]. |
| Marketing Status |
approved; investigational |
| ATC Code |
L01XH04 |
| DrugBank ID |
DB05015
|
| KEGG ID |
D08870
|
| MeSH ID |
C487081
|
| PubChem ID |
6918638
|
| TTD Drug ID |
D0XT6W
|
| NDC Product Code |
65392-2509; 54893-0074; 72893-002 |
| UNII |
F4H96P17NZ
|
| Synonyms |
belinostat | Belecodaq | PXD101 |
|
| Chemical Information |
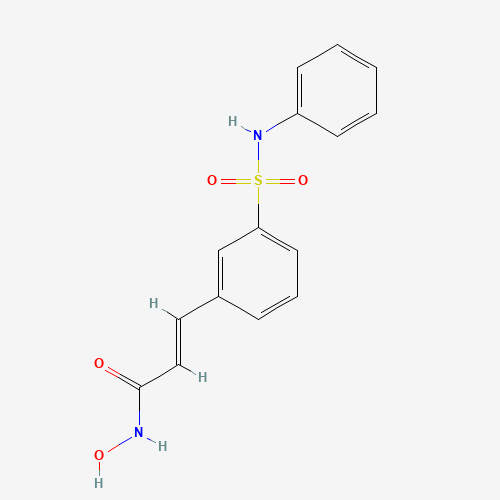
| Molecular Formula |
C15H14N2O4S |
| CAS Registry Number |
866323-14-0 |
| SMILES |
C1=CC=C(C=C1)NS(=O)(=O)C2=CC=CC(=C2)C=CC(=O)NO |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

