| Pharmaceutical Information |
| Drug Name |
Aripiprazole lauroxil |
| Drug ID |
BADD_D00166 |
| Description |
Aripiprazole lauroxil is a long-acting injectable atypical antipsychotic drug used in the treatment of schizophrenia in adult patients. It is a prodrug of [aripiprazole], which acts as a partial agonist at the D2 and 5-HT1A receptors, and as an antagonist at the 5-HT2A receptors [A34289].
Affecting about 1% of the adult population in the United States and approximately 26 million people worldwide, schizophrenia is a chronic neurological disorder that may result in impairments in cognition and executive functions [A34289]. The quality of life in patients is greatly reduced due to negative health outcomes, and oftentimes the patients are faced with social stigma and discriminations. Schizophrenia is characterized by positive symptoms such as delusions, hallucinations, thought disorders, and catanoia, and negative symptoms that include social withdrawal, anhedonia, and flattening of emotional responses [T28]. D2 receptors have been the most common target for antipsychotic agents used in the treatment of schizophrenia: the positive symptoms are thought to arise from overactivity in the mesolimbic dopaminergic pathway activating D2 receptors, whereas negative symptoms may result from a decreased activity in the mesocortical dopaminergic pathway with D1 receptors predominating [T28]. In a randomized, double-blind clinical trial, treatment of aripiprazole lauroxil in adult patients with schizophrenia resulted in improvement of positive and negative symptoms scores at day 85 of treatment [A34289, A34301].
Aripiprazole lauroxil was initially approved by the FDA in October 2015 under the market name Aristada for the treatment of schizophrenia. It is administered via intramuscular injection, and requires the establishment of tolerability prior to dosing in treatment-naïve patients [FDA Label]. On July 2nd, a different formulation of aripiprazole lauroxil marketed as Aristada Initio was FDA-approved for immediate initiation of Aristada at any dose. The patients may receive Aristada Initio in combination with a single 30 mg oral dose of aripiprazole to achieve appropriate levels of aripiprazole more rapidly. Long-acting injectable aripiprazole lauroxil displayed comparable efficacy and safety to aripiprazole [A34301], and reduced dosing frequency improves patient adherence. |
| Indications and Usage |
Aripiprazole lauroxil is indicated for the treatment of schizophrenia and related psychotic disorders. |
| Marketing Status |
approved; investigational |
| ATC Code |
Not Available |
| DrugBank ID |
DB14185
|
| KEGG ID |
D10364
|
| MeSH ID |
C000603935
|
| PubChem ID |
49831411
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
12658-0531; 58032-1020; 65757-403; 12658-0491; 12658-0530; 65757-404; 65757-500; 12658-0535; 12658-0569; 59116-5070; 64552-4079; 12658-0536; 65757-402; 12658-0537; 12658-0597; 65757-401; 69037-0045; 12658-0490; 12658-0532 |
| UNII |
B786J7A343
|
| Synonyms |
aripiprazole lauroxil | Aristada |
|
| Chemical Information |
| Molecular Formula |
C36H51Cl2N3O4 |
| CAS Registry Number |
1259305-29-7 |
| SMILES |
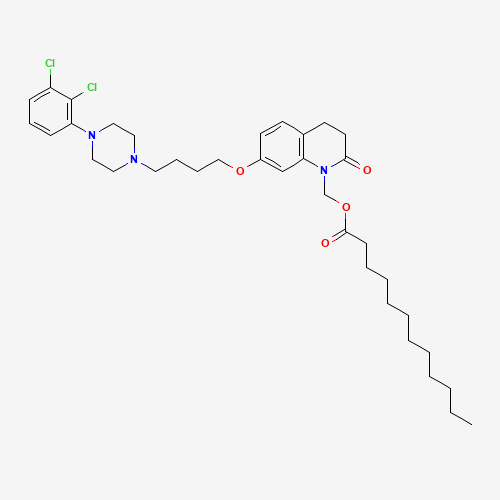
CCCCCCCCCCCC(=O)OCN1C(=O)CCC2=C1C=C(C=C2)OCCCCN3CCN(CC3)C4=C(C(=CC=C4)Cl)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

