| Pharmaceutical Information |
| Drug Name |
Amlodipine besylate |
| Drug ID |
BADD_D00122 |
| Description |
Amlodipine, initially approved by the FDA in 1987, is a popular antihypertensive drug belonging to the group of drugs called _dihydropyridine calcium channel blockers_. Due to their selectivity for the peripheral blood vessels, dihydropyridine calcium channel blockers are associated with a lower incidence of myocardial depression and cardiac conduction abnormalities than other calcium channel blockers [A175327].
Amlodipine is commonly used in the treatment of high blood pressure and angina. Amlodipine has antioxidant properties and an ability to enhance the production of nitric oxide (NO), an important vasodilator that decreases blood pressure [A175321]. The option for single daily dosing of amlodipine is an attractive feature of this drug [FDA label]. |
| Indications and Usage |
Amlodipine may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of the following conditions [FDA label]:
• Hypertension
• Coronary artery disease
• Chronic stable angina
• Vasospastic angina (Prinzmetal’s or Variant angina)
• Angiographically documented coronary artery disease in patients without heart failure or an ejection fraction < 40% |
| Marketing Status |
approved |
| ATC Code |
C08CA01 |
| DrugBank ID |
DB00381
|
| KEGG ID |
D00615
|
| MeSH ID |
D017311
|
| PubChem ID |
60496
|
| TTD Drug ID |
D08JIV
|
| NDC Product Code |
76282-237; 82009-026; 10135-761; 55111-025; 58159-068; 65862-103; 43063-764; 51655-291; 58118-1127; 68071-2312; 0480-7168; 70518-1971; 70934-330; 71205-488; 71205-506; 71335-0890; 71335-1597; 76282-238; 38779-2734; 57451-1188; 68981-016; 42708-066; 43063-041; 50090-1627; 50090-2524; 50090-4903; 63187-198; 63187-508; 63187-914; 68071-3453; 68382-122; 68645-580; 70934-075; 71093-146; 71335-0934; 71335-0945; 71335-1887; 72789-260; 76282-507; 65427-003; 66174-0020; 29300-397; 51655-586; 52605-041; 52605-042; 53002-3351; 55700-543; 60687-496; 61919-844; 68071-2270; 68071-2305; 68788-6386; 69097-126; 70518-0274; 70518-0835; 71335-0843; 0615-8282; 55700-541; 63187-197; 65841-620; 68071-2415; 68645-516; 68788-7679; 68788-7985; 68788-8313; 0480-0083; 69584-022; 70518-3592; 71093-147; 71093-148; 71335-0218; 0615-8283; 0615-8429; 72789-082; 76282-509; 0904-6369; 53104-7561; 10135-759; 65977-0005; 68724-0008; 72761-034; 43063-564; 52605-043; 55154-6875; 59762-2135; 60760-459; 60760-614; 61919-753; 61919-782; 67877-198; 68645-515; 71335-1278; 71610-691; 72789-261; 0904-6370; 52932-0723; 65691-0040; 42806-057; 53002-1249; 61919-805; 63629-1023; 67296-1876; 67877-197; 68071-4251; 69097-128; 71205-599; 71335-0833; 71335-1032; 71610-620; 0615-8284; 0615-8430; 76282-239; 51552-1438; 0069-1530; 57218-936; 42806-056; 43353-989; 53002-2352; 53808-1100; 65162-008; 65841-621; 68382-123; 68788-9345; 69097-836; 69584-021; 69584-023; 72789-262; 42185-7058; 53747-011; 65862-102; 29300-396; 29300-398; 42291-025; 42291-026; 55154-6892; 59762-2242; 60687-488; 61919-814; 65162-006; 65862-101; 68180-720; 68180-721; 68788-8263; 69097-838; 70518-2632; 72189-162; 65015-670; 43353-684; 55154-8093; 59762-2010; 60760-445; 63629-1024; 65162-007; 65841-622; 67296-1530; 67877-199; 68382-121; 68788-8451; 69097-837; 0480-7167; 70934-920; 71610-469; 71610-539; 71610-687; 82009-028; 49452-0429; 0069-1520; 10135-760; 64220-183; 42806-055; 63187-326; 63187-783; 67296-1529; 68180-719; 69097-127; 70518-2981; 71205-596; 71335-0473; 0615-8431; 76282-508; 82009-027; 82982-020; 0904-6371; 51927-0073; 0069-1540; 65862-344; 71052-266; 71859-001; 42291-027; 43353-691; 50090-2581; 53002-1351; 53002-1352 |
| UNII |
864V2Q084H
|
| Synonyms |
Amlodipine | Amlodipine, (+-)-Isomer | Amlodipine Besylate | Amlodipine, (+-)-Isomer, Maleate (1:1) | Amlodipine, (S)-Isomer, Maleate (1:1) | Amlodis | Astudal | Norvasc | Istin | Amlor | Amlodipine Maleate | Amlodipine Maleate (1:1) | Amlodipine, (R)-Isomer |
|
| Chemical Information |
| Molecular Formula |
C26H31ClN2O8S |
| CAS Registry Number |
111470-99-6 |
| SMILES |
CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN.C1=CC=C(C=C1)S(=O)(=O)O |
| Chemical Structure |
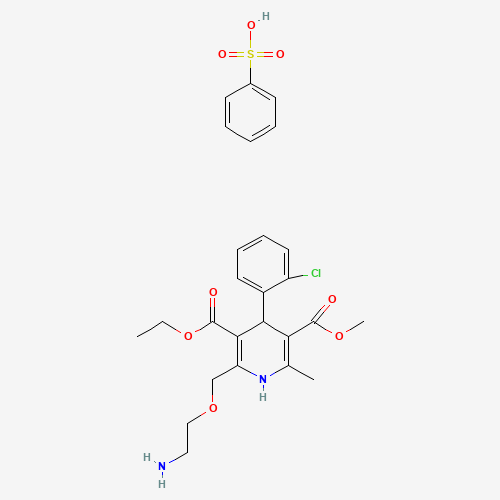
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
|

