| Pharmaceutical Information |
| Drug Name |
Alendronic acid |
| Drug ID |
BADD_D00066 |
| Description |
Alendronic acid is a second generation bisphosphonate that is used for the treatment of some forms of osteoperosis and Paget's disease[FDA Label][A959,A203111]. It functions by preventing resorption of bone[FDA Label][A959]. |
| Indications and Usage |
Alendronic acid is indicated for the treatment and prevention of osteoporosis in men and postmenopausal women, treatment of glucocorticoid-induced osteoporosis, and Paget's disease of bone[FDA Label][A959,A176750]. However, alendronic acid is not indicated for use in pediatric populations or patients with a creatinine clearance <35mL/min[FDA Label]. |
| Marketing Status |
approved |
| ATC Code |
M05BA04 |
| DrugBank ID |
DB00630
|
| KEGG ID |
D07119
|
| MeSH ID |
D019386
|
| PubChem ID |
2088
|
| TTD Drug ID |
D09KLR
|
| NDC Product Code |
Not Available |
| UNII |
X1J18R4W8P
|
| Synonyms |
Alendronate | 4-Amino-1-Hydroxybutylidene 1,1-Biphosphonate | Aminohydroxybutane Bisphosphonate | MK-217 | MK 217 | MK217 | Alendronate Monosodium Salt, Trihydrate | Alendronate Sodium | Fosamax |
|
| Chemical Information |
| Molecular Formula |
C4H13NO7P2 |
| CAS Registry Number |
66376-36-1 |
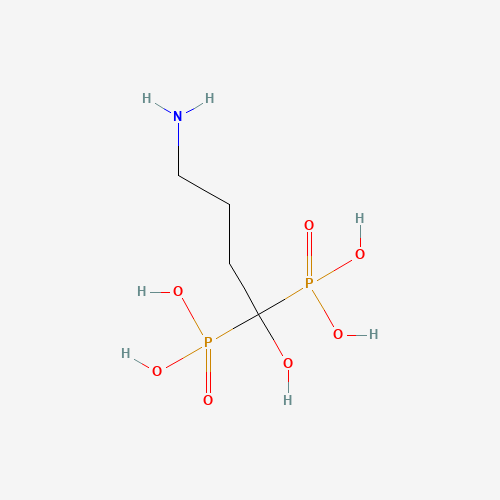
| SMILES |
C(CC(O)(P(=O)(O)O)P(=O)(O)O)CN |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Gingival atrophy | 07.09.13.017 | - | - | - |
|
|
|

