| Pharmaceutical Information |
| Drug Name |
Povidone iodine |
| Drug ID |
BADD_D01813 |
| Description |
Povidone-iodine is a stable chemical complex of polyvinylpyrrolidone (povidone, PVP) and elemental iodine. It contains from 9.0% to 12.0% available iodine, calculated on a dry basis. This unique complex was discovered in 1955 at the Industrial Toxicology Laboratories in Philadelphia by H. A. Shelanski and M. V. Shelanski. During in vitro testing to demonstrate anti-bacterial activity it was found that the complex was less toxic in mice than tincture of iodine. Human clinical trials showed the product to be superior to other iodine formulations. Povidone-iodine was immediately marketed, and has since become the universally preferred iodine antiseptic. |
| Indications and Usage |
For topical application in the treatment and prevention of infection in wounds. |
| Marketing Status |
approved |
| ATC Code |
S01AX18; D11AC06; G01AX11; D08AG02; D09AA09; R02AA15 |
| DrugBank ID |
DB06812
|
| KEGG ID |
D00863
|
| MeSH ID |
D011206
|
| PubChem ID |
410087
|
| TTD Drug ID |
D03EXK
|
| NDC Product Code |
10819-3890; 34645-4020; 50425-011; 60913-051; 63517-132; 34645-1029; 68786-132; 69842-935; 0904-1103; 34645-1043; 34645-4059; 34645-4009; 50425-010; 34645-1028; 34645-1044; 60913-999; 34645-1030; 34645-1031; 69842-325; 25119-3000; 34645-1032; 34645-4188; 52380-0038; 65517-0036; 68599-3501; 70115-0004; 34645-4010 |
| UNII |
Not Available
|
| Synonyms |
Povidone-Iodine | Povidone Iodine | Povidone-Iodines | PVP-I | PVP-Iodine | PVP Iodine | PVP-Iodines | Polyvinylpyrrolidone Iodine | Polyvinylpyrrolidone Iodines | Betadine | Betadines | Providine | Providines | Disadine | Disadines | Isodine | Isodines | Pharmadine | Pharmadines | Alphadine | Alphadines | Betaisodona |
|
| Chemical Information |
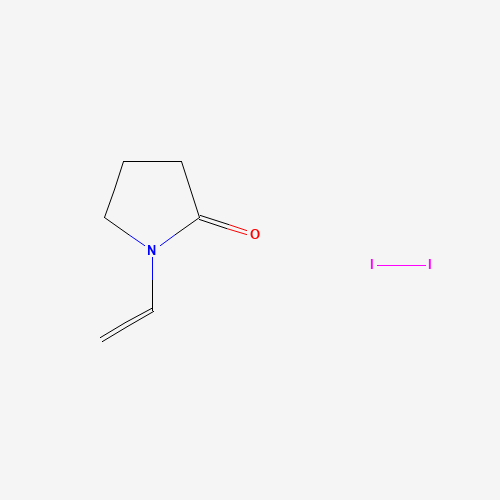
| Molecular Formula |
C6H9I2NO |
| CAS Registry Number |
25655-41-8 |
| SMILES |
C=CN1CCCC1=O.II |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice.
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Erythema | 23.03.06.001 | - | - | - | | Hypersensitivity | 10.01.03.003 | - | - | | | Skin irritation | 23.03.04.009 | - | - | - |
|
The 1th Page
1
Total 1 Pages
|
|

